
Concept explainers
(a)
Interpretation:
The resulting alcohol should be identified after reacting following alkene with

Concept Introduction:
Reaction of an alkene with
The hydration reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 56P
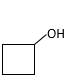
Explanation of Solution
Unsaturated (
(b)
Interpretation:
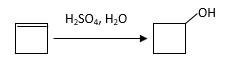
The resulting alcohol should be identified after reacting following alkene with
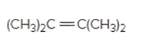
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Hydration reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 56P
Explanation of Solution
Unsaturated (
(c)
Interpretation:
The resulting alcohol should be identified after reacting following alkene with
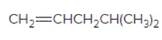
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Hydration reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 56P
Explanation of Solution
Unsaturated (
Refer to the below reaction:
(d)
Interpretation:
The resulting alcohol should be identified after reacting following alkene with
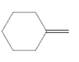
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
Reaction of an alkene with
Hydration reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 56P
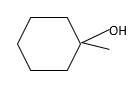
Explanation of Solution
Unsaturated (
Refer to the below reaction:
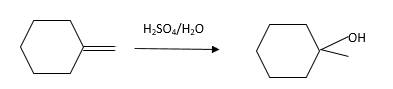
Want to see more full solutions like this?
Chapter 13 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
- In GC, what order will the following molecules elute from the column? CH3OCH3, CH3CH2OH, C3H8, C4H10arrow_forwardBeer’s Law is A = εbc, where A is absorbance, ε is the molar absorptivity (which is specific to the compound and wavelength in the measurement), and c is concentration. The absorbance of a 2.31 × 10-5 M solution of a compound is 0.822 at a wavelength of 266 nm in a 1.00-cm cell. Calculate the molar absorptivity at 266 nm.arrow_forwardHow to calculate % of unknown solution using line of best fit y=0.1227x + 0.0292 (y=2.244)arrow_forward
- Given a 1,3-dicarbonyl compound, state the (condensed) formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardComplete the following acid-base reactions and predict the direction of equilibrium for each. Justify your prediction by citing pK values for the acid and conjugate acid in each equilibrium. (a) (b) NHs (c) O₂N NH NH OH H₁PO₁arrow_forward23.34 Show how to convert each starting material into isobutylamine in good yield. ཅ ནད ཀྱི (b) Br OEt (c) (d) (e) (f) Harrow_forward
- Please help me Please use https://app.molview.com/ to draw this. I tried, but I couldn't figure out how to do it.arrow_forwardPropose a synthesis of 1-butanamine from the following: (a) a chloroalkane of three carbons (b) a chloroalkane of four carbonsarrow_forwardSelect the stronger base from each pair of compounds. (a) H₂CNH₂ or EtzN (b) CI or NH2 NH2 (c) .Q or EtzN (d) or (e) N or (f) H or Harrow_forward
- 4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forwardb. H3C CH3 H3O+ ✓ H OHarrow_forward2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



