
(a)
Interpretation:
Enol tautomer of 2,4-pentanedione has to be drawn.
Concept Introduction:
Tautomerism is the ability of a molecule to exist in more than one chemical form. Tautomers are formed by the migration of a hydrogen atom, accompanied by the switching of a single and neighboring double bond.
The only difference in keto-enol tautomers is the location of hydrogen and double bond.
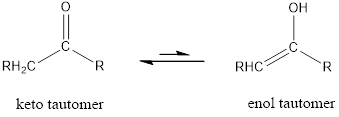
(b)
Interpretation:
Reason for most
Concept Introduction:
Tautomerism is the ability of a molecule to exist in more than one chemical form. Tautomers are formed by the migration of a hydrogen atom, accompanied by the switching of a single and neighboring double bond.
The only difference in keto-enol tautomers is the location of hydrogen and double bond.

Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- Can I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forward
- I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
