
EBK ESSENTIAL ORGANIC CHEMISTRY
3rd Edition
ISBN: 8220100659461
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 31P
Interpretation Introduction
Interpretation:
The product of the given reaction has to be determined.
Concept introduction:
Aldol addition is an reaction which occurs between two molecules of
One molecule (aldehyde or ketone) acts a nucleophile and attacks the electrophilic carbon center of the other molecule to give the addition product. The product is named
Mechanism for the aldol addition:
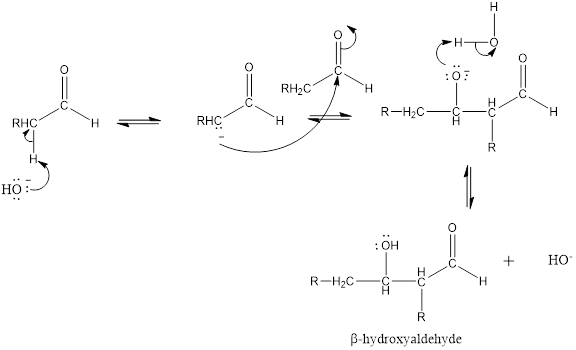
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Identifying the stereochemistry of natural
Write the complete common (not IUPAC) name of each molecule below.
Note: if a molecule is one of a pair of enantiomers, be sure you start its name with D- or L- so we know which enantiomer it is.
molecule
H
O-C-CH2
H3N.
HN
N
H
C=O
common name
(not the IUPAC
name)
NH3
☐
H3N
H
☐
CH2
X
>
Draw the structure of alanine at pH 1.2.
Click and drag to start drawing a
structure.
Understanding the general acid-base properties of amino acids
O Proteins
Imagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or
neutral?
molecule
The solution is...
010
H3N-CH-C-OH
CH
HO
CH3
O acidic
O basic
neutral
O (unknown)
H3N
HO
0
O acidic
O basic
neutral
○ (unknown)
H3N-CH-C-O
CH2
CH3-CH-CH3
O acidic
O basic
Oneutral
○ (unknown)
O=
X
H2N-CH-C-O
CH3
CH
CH3
acidic
O basic
O neutral
○ (unknown)
?
000
Chapter 13 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
Ch. 13.1 - Identify the most acidic hydrogen in each...Ch. 13.1 - Prob. 2PCh. 13.1 - Prob. 3PCh. 13.1 - Prob. 4PCh. 13.1 - Explain why HO cannot remove a proton from the...Ch. 13.2 - Prob. 6PCh. 13.2 - Prob. 7PCh. 13.3 - Prob. 8PCh. 13.3 - Prob. 9PCh. 13.3 - Prob. 10P
Ch. 13.4 - Prob. 11PCh. 13.5 - Prob. 12PCh. 13.5 - Prob. 13PCh. 13.6 - Prob. 14PCh. 13.7 - Prob. 16PCh. 13.8 - Prob. 17PCh. 13.8 - Prob. 18PCh. 13.8 - Prob. 19PCh. 13.9 - Prob. 20PCh. 13.10 - Propose a mechanism for the formation of...Ch. 13.10 - Prob. 22PCh. 13.10 - a. If the biosynthesis of palmitic acid were...Ch. 13 - Draw the enol tautomers for each of the following...Ch. 13 - Number the following compounds in order from...Ch. 13 - Prob. 26PCh. 13 - Explain why the pKa of a hydrogen bonded to the...Ch. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - Prob. 31PCh. 13 - Prob. 32PCh. 13 - Prob. 33PCh. 13 - Using cyclopentanone as the reactant, show the...Ch. 13 - Prob. 35PCh. 13 - Prob. 36PCh. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - Prob. 44PCh. 13 - Describe how the following compounds can be...Ch. 13 - Prob. 46PCh. 13 - Which would require a higher temperature:...Ch. 13 - Prob. 48PCh. 13 - Propose a mechanism for the following reaction:Ch. 13 - Show how the following compounds could be...Ch. 13 - Prob. 51PCh. 13 - Prob. 52P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Imagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule 0=0 H3N-CH-C-o HO CH2 OH The solution is... O acidic O basic O neutral O (unknown) H₂N acidic O basic O neutral ○ (unknown) + H3N O OH O acidic O basic O neutral O (unknown) H2N-CH-C-O CH3 O acidic O basic neutral ○ (unknown) X ? olo HEarrow_forwardRecognizing ampli Draw an a amino acid with a methyl (-CH3) side chain. Explanation Check Click and drag to start drawing a structure. X Carrow_forwardWrite the systematic name of each organic molecule: structure name × HO OH ☐ OH CI CI O CI OH OHarrow_forward
- く Check the box under each a amino acid. If there are no a amino acids at all, check the "none of them" box under the table. Note for advanced students: don't assume every amino acid shown must be found in nature. COO H3N-C-H CH2 HO CH3 NH3 O CH3-CH CH2 OH Onone of them Explanation Check + H3N O 0. O OH + NH3 CH2 CH3-CH H2N C-COOH H O HIC + C=O H3N-C-O CH3- - CH CH2 OH Х 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardWrite the systematic name of each organic molecule: structure HO-C-CH2-CH3 O -OH CH3-CH2-CH2-CH2-CH2-C-OH CH3 CH3-CH-CH2-C-OH Explanation Check S namearrow_forwardtheres 2 productsarrow_forward
- Draw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forwardDraw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forwardOrganic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forward
- Differentiate between electrophilic and nucleophilic groups. Give examples.arrow_forwardAn aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forwardDraw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT