
Concept explainers
Interpretation:
The compound, which reacts faster with sodium methoxide in methanol in each of the given pair of compounds is to be determined and the chemical equation for the faster reaction is to be written.
Concept introduction:
Nucleophilic
In nucleophilic aromatic substitution reactions, the nucleophile substitutes a leaving group from the aryl ring.
Aryl halides bearing an electron withdrawing substituent undergo nucleophilic substitution rapidly.
The substituents attached ortho and para with respect to the halogen atom in the aryl halide react at similar rates. The substituents attached at meta position in the aryl halide react at slower rates than ortho and para substituents.
Electron withdrawing substituents stabilize the intermediate carbanion formed and thus are strongly activating substituents in the nucleophilic aromatic substitution reactions.
Electron donating substituents destabilize the intermediate carbanion formed and thus are strongly deactivating substituents in the nucleophilic aromatic substitution reactions.
Answer to Problem 43P
Solution:
a)
The reaction is as follows:
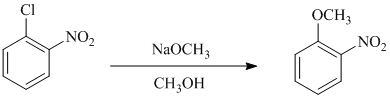
b) In between
The reaction is as follows:

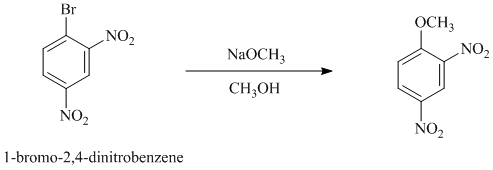
c) In between
The reaction is as follows:
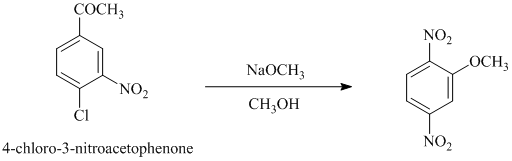
d) In between
The reaction is as follows:
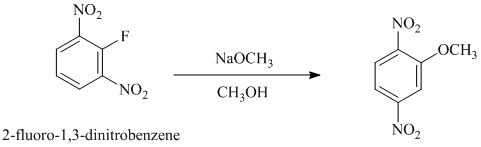
e) In between
The reaction is as follows:
Explanation of Solution
a)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
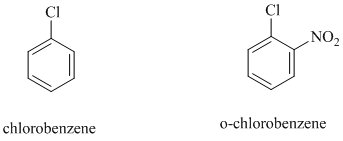
In chlorobenzene, a chlorine atom is attached to the benzene ring while in
The reaction of
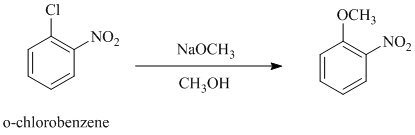
b)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
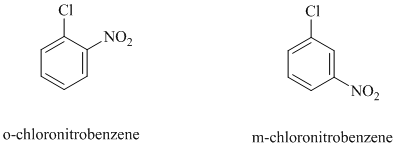
In both the given aryl halides, a strong electron withdrawing substituent is attached on the ring. In
The reaction of
c)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
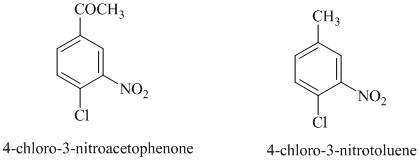
In
In
Thus, in between
The reaction of

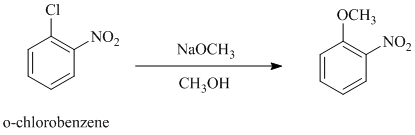
d)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
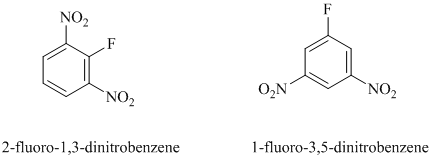
Nitro substituents are strong electron withdrawing substituents.
In
Electron withdrawing substituents at ortho and para positions activate the ring more than the electron withdrawing substituents at meta positions.
Thus, in between
The reaction of

e)
In this nucleophilic aromatic substitution reaction, sodium methoxide is a source of the nucleophile
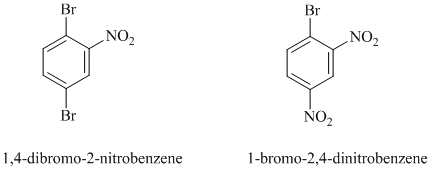
In
The reaction of

Want to see more full solutions like this?
Chapter 13 Solutions
CAREY: ORGANIC CHEMISTRY
- Protecting Groups and Carbonyls 6) The synthesis generates allethrolone that exhibits high insect toxicity but low mammalian toxicity. They are used in pet shampoo, human lice shampoo, and industrial sprays for insects and mosquitos. Propose detailed mechanistic steps to generate the allethrolone label the different types of reagents (Grignard, acid/base protonation, acid/base deprotonation, reduction, oxidation, witting, aldol condensation, Robinson annulation, etc.) III + VI HS HS H+ CH,CH,Li III I II IV CI + P(Ph)3 V ༼ Hint: no strong base added VI S VII IX HO VIII -MgBr HgCl2,HgO HO. isomerization aqeuous solution H,SO, ༽༽༤༽༽ X MeOH Hint: enhances selectivity for reaction at the S X ☑arrow_forwardDraw the complete mechanism for the acid-catalyzed hydration of this alkene. esc 田 Explanation Check 1 888 Q A slock Add/Remove step Q F4 F5 F6 A བྲA F7 $ % 5 @ 4 2 3 & 6 87 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce W E R T Y U S D LL G H IK DD 요 F8 F9 F10 F1 * ( 8 9 0 O P J K L Z X C V B N M H He commandarrow_forwardExplanation Check F1 H₂O H₂ Pd 1) MCPBA 2) H3O+ 1) Hg(OAc)2, H₂O 2) NaBH4 OH CI OH OH OH hydration halohydrin formation addition halogenation hydrogenation inhalation hydrogenation hydration ☐ halohydrin formation addition halogenation formation chelation hydrogenation halohydrin formation substitution hydration halogenation addition Ohalohydrin formation subtraction halogenation addition hydrogenation hydration F2 80 F3 σ F4 F5 F6 1 ! 2 # 3 $ 4 % 05 Q W & Å © 2025 McGraw Hill LLC. All Rights Reserved. F7 F8 ( 6 7 8 9 LU E R T Y U A F9arrow_forward
- Show the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forwardSoap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
- The two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forwardасть Identify all the bonds that gauche interact with C-OMe in the most stable conformation of the above compound.arrow_forwardPredict the reactants used in the formation of the following compounds using Acid-Catalyzed dehydration reactionarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


