
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 36P
Starting with cyclohexane, how could the following compounds be prepared?


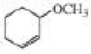
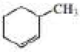
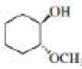
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
11. Help
8. Help
9. Help
Chapter 13 Solutions
Organic Chemistry
Ch. 13.2 - Prob. 1PCh. 13.2 - Write the steps for formation of...Ch. 13.3 - Prob. 3PCh. 13.4 - Prob. 4PCh. 13.5 - Prob. 7PCh. 13.5 - a. Would chlorination or bromination produce a...Ch. 13.5 - Prob. 10PCh. 13.6 - Prob. 11PCh. 13.7 - Prob. 12PCh. 13.7 - Prob. 13P
Ch. 13.8 - Prob. 14PCh. 13.8 - Draw the stereoisomers of the major...Ch. 13.9 - a. How many stereoisomers are formed from the...Ch. 13.9 - Prob. 17PCh. 13.9 - Prob. 19PCh. 13.9 - Prob. 20PCh. 13.9 - Prob. 21PCh. 13.10 - Prob. 22PCh. 13.11 - How many atoms share the unpaired electrons in...Ch. 13.11 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - Prob. 26PCh. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - Prob. 31PCh. 13 - Prob. 32PCh. 13 - Prob. 33PCh. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Starting with cyclohexane, how could the following...Ch. 13 - a. Propose a mechanism for the following reaction:...Ch. 13 - What stereoisomers are obtained from the following...Ch. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Draw the products of the following reactions,...Ch. 13 - a. What five-carbon alkene forms the same product...Ch. 13 - Prob. 44PCh. 13 - Prob. 45PCh. 13 - Prob. 46PCh. 13 - Explain why the rate of bromination of methane...Ch. 13 - Prob. 48P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY