
Chemistry for Today: General Organic and Biochemistry
9th Edition
ISBN: 9781337514576
Author: Seager
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 13, Problem 13.30E
Each of the following conversions requires more than one step, and some reactions studied in previous chapters may be needed. Show the reagents you would use and draw structural formulas for intermediate compounds formed in each conversion.
a. 
b. 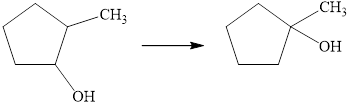
c. 
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Vibrational contributions to internal energy and heat capacity1) are temperature independent2) are temperature dependent
The approximation of calculating the partition function by integration instead of the summation of all the energy terms can only be done if the separation of the energy levels is much smaller than the product kT. Explain why.
Explain the meaning of: the electron partition function is equal to the degeneracy of the ground state.
Chapter 13 Solutions
Chemistry for Today: General Organic and Biochemistry
Ch. 13 - Draw general formulas for an alcohol and phenol,...Ch. 13 - Prob. 13.2ECh. 13 - Assign IUPAC names to the following alcohols: a....Ch. 13 - Assign IUPAC names to the following alcohols: a....Ch. 13 - Several important alcohols are well known by...Ch. 13 - Prob. 13.6ECh. 13 - Draw structural formulas for each of the...Ch. 13 - Draw structural formulas for each of the...Ch. 13 - Name each of the following as a derivative of...Ch. 13 - Name each of the following as a derivative of...
Ch. 13 - Prob. 13.11ECh. 13 - Draw structural formulas for each of the...Ch. 13 - Prob. 13.13ECh. 13 - Classify the following alcohols as primary,...Ch. 13 - Classify the following alcohols as primary,...Ch. 13 - Draw structural formulas for the four aliphatic...Ch. 13 - Why are the boiling points of alcohols much higher...Ch. 13 - Arrange the compounds of each group in order of...Ch. 13 - Prob. 13.19ECh. 13 - Draw structural formulas for the following...Ch. 13 - Prob. 13.21ECh. 13 - Draw the structures of the chief product formed...Ch. 13 - Draw the structures of the chief product formed...Ch. 13 - Draw the structures of the ethers that can be...Ch. 13 - Prob. 13.25ECh. 13 - Give the structure of an alcohol that could be...Ch. 13 - Give the structure of an alcohol that could be...Ch. 13 - What products would result from the following...Ch. 13 - What products would result from the following...Ch. 13 - Each of the following conversions requires more...Ch. 13 - Each of the following conversions requires more...Ch. 13 - The three-carbon diol used in antifreeze is It is...Ch. 13 - Methanol is fairly volatile and evaporates quickly...Ch. 13 - Prob. 13.34ECh. 13 - Prob. 13.35ECh. 13 - Name an alcohol used in each of the following...Ch. 13 - Prob. 13.37ECh. 13 - Prob. 13.38ECh. 13 - Assign a common name to each of the following...Ch. 13 - Assign a common name to each of the following...Ch. 13 - Assign the IUPAC name to each of the following...Ch. 13 - Assign the IUPAC name to each of the following...Ch. 13 - Prob. 13.43ECh. 13 - Draw structural formulas for the following: a....Ch. 13 - Prob. 13.45ECh. 13 - Prob. 13.46ECh. 13 - Prob. 13.47ECh. 13 - Arrange the following compounds in order of...Ch. 13 - Arrange the compounds in Exercise 13.48 in order...Ch. 13 - Prob. 13.50ECh. 13 - Complete the following reactions: a. b....Ch. 13 - Prob. 13.52ECh. 13 - Lipoic acid is required by many microorganisms for...Ch. 13 - Alcohols and thiols can both be oxidized in a...Ch. 13 - Prob. 13.55ECh. 13 - Prob. 13.56ECh. 13 - Prob. 13.57ECh. 13 - Thiols have lower boiling points and are less...Ch. 13 - Prob. 13.59ECh. 13 - Prob. 13.60ECh. 13 - Prob. 13.61ECh. 13 - Prob. 13.62ECh. 13 - A mixture of ethanol and 1propanol is heated to...Ch. 13 - Prob. 13.64ECh. 13 - Prob. 13.65ECh. 13 - Prob. 13.66ECh. 13 - Prob. 13.67ECh. 13 - Figure 13.8 points out that methanol is used as a...Ch. 13 - Figure 13.13 focuses on the use of thiol chemistry...Ch. 13 - Prob. 13.70ECh. 13 - Prob. 13.71ECh. 13 - Prob. 13.72ECh. 13 - The compound that has the greatest polarity is: a....Ch. 13 - Alcoholic beverages contain: a. wood alcohol. b....Ch. 13 - Prob. 13.75ECh. 13 - Which of the following compounds is an ether? a....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 28. For each of the following species, add charges wherever required to give a complete, correct Lewis structure. All bonds and nonbonded valence electrons are shown. a. b. H H H H H :0-C-H H H H-C-H C. H H d. H-N-0: e. H H-O H-O H B=0 f. H—Ö—Ñ—Ö—H Norton Private Barrow_forwardAt 0oC and 1 atm, the viscosity of hydrogen (gas) is 8.55x10-5 P. Calculate the viscosity of a gas, if possible, consisting of deuterium. Assume that the molecular sizes are equal.arrow_forwardIndicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forward
- Indicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardDraw the skeletal structure of the alkane 4-ethyl-2, 2, 5, 5- tetramethylnonane. How many primary, secondary, tertiary, and quantenary carbons does it have?arrow_forwardDon't used Ai solutionarrow_forward
- Don't used Ai solutionarrow_forwardThe number of imaginary replicas of a system of N particlesA) can never become infiniteB) can become infiniteC) cannot be greater than Avogadro's numberD) is always greater than Avogadro's number.arrow_forwardElectronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forward
- Please correct answer and don't used hand raitingarrow_forwardCalculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forwardGeneral formula etherarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY