
(a)
Interpretation:
An IUPAC name for the alcohol,
Concept introduction:
The systematic naming of organic compound is given by
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene, -ol, -al, -oic and so on.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 13.6E
The IUPAC name for the alcohol,
Explanation of Solution
In
The IUPAC name for the alcohol,
(b)
Interpretation:
An IUPAC name for the given alcohol is to be predicted.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene, -ol, -al, -oic and so on.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 13.6E
An IUPAC name for the given alcohol is propan-
Explanation of Solution
The alcohol for which the IUPAC name has to be predicted is shown below.

Figure 1
The longest carbon chain in this alcohol is made up of three carbon atoms. The three carbon atoms chain makes this alcohol a propanol. The hydroxyl group is attached to the second carbon atom. Therefore, the IUPAC name for this alcohol is propan-
An IUPAC name for the given alcohol is propan-
(c)
Interpretation:
An IUPAC name for the alcohol,
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene, -ol, -al, -oic and so on.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 13.6E
An IUPAC name for the alcohol,
Explanation of Solution
In
An IUPAC name for the alcohol,
(d)
Interpretation:
An IUPAC name for the given alcohol is to be predicted.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene, -ol, -al, -oic and so on.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 13.6E
An IUPAC name for the given alcohol is
Explanation of Solution
The alcohol for which the IUPAC name has to be predicted is shown below.
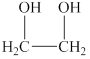
Figure 2
The longest carbon chain in this alcohol is made up of two carbon atoms. The hydroxyl groups are attached to the first and second carbon atom. Therefore, the IUPAC name for the given alcohol is
An IUPAC name for the given alcohol is
(e)
Interpretation:
An IUPAC name for the given alcohol is to be predicted.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene, -ol, -al, -oic and so on.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 13.6E
An IUPAC name for the given alcohol is propane-
Explanation of Solution
The alcohol for which the IUPAC name has to be predicted is shown below.
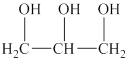
Figure 3
The longest carbon chain in this alcohol is made up of three carbon atoms. One hydroxyl group is attached to each carbon atom. Therefore, the alcohol will be propanetriol. The hydroxyl group is attached to the first, second and third carbon atom. Therefore, the IUPAC name for the given alcohol is propane-
An IUPAC name for the given alcohol is propane-
Want to see more full solutions like this?
Chapter 13 Solutions
Chemistry for Today: General Organic and Biochemistry
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning



