
What products would result from the following processes?
Write an equation for each reaction.
a.
b.
c.
d.
e.
(a)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.29E
The product formed when
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
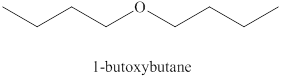
Alcohols on heating at
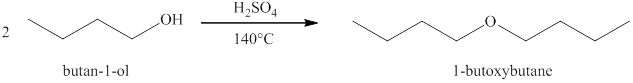
Figure 1
The product formed when
(b)
Interpretation:
The product formed by the excess oxidation of
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.29E
The product formed by the excess oxidation of
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
The primary alcohol,

Figure 2
The product formed by the excess oxidation of
(c)
Interpretation:
The product formed by the controlled oxidation of
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.29E
The product formed when
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
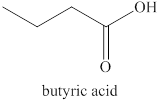
The compound
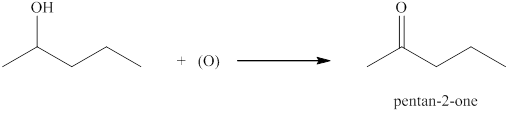
Figure 3
The product formed when
(d)
Interpretation:
The product formed when
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.29E
The product formed when
![]()
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
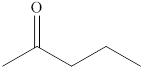
Alcohols on heating at temperature
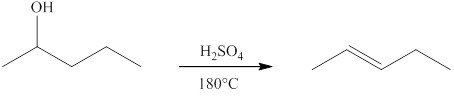
Figure 4
Interpretation:
The product formed when
(e)
Interpretation:
The product formed by the controlled oxidation of
Concept introduction:
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
Answer to Problem 13.29E
No product is formed by the controlled oxidation of
Explanation of Solution
Ketones can be prepared by the oxidation reaction of secondary alcohol. On the other hand, primary alcohols on oxidation with weak oxidant give aldehyde and with strong oxidant give carboxylic acid. No product is obtained by the oxidation of tertiary alcohol.
The controlled oxidation of
No product is formed by the controlled oxidation of
Want to see more full solutions like this?
Chapter 13 Solutions
Chemistry for Today: General Organic and Biochemistry
- For each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label each compound as chiral or achiral.arrow_forwardBlackboard app.aktiv.com X Organic Chemistry II Lecture (mx Aktiv Learning App Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 25 of 35 Select to Edit Arrows CH3CH2OK, CH3CH2OH L Gemini M 31 0:0 :0: 5x Undo Reset Done :0: Harrow_forwardI have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to me.I have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to marrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. Problem 17 of 35 1. CH3CH2Li O H 2. Neutralizing work-up @ Atoms, Bonds and Rings Draw or tap a new boarrow_forwardWill this convert the C=O to an alcohol? Or does its participation in the carboxy group prevent that from happening?arrow_forwardI have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to me.I have some reactions here for which I need to predict the products. Can you help me solve them and rewrite the equations, as well as identify the type of reaction? Please explain it to marrow_forward
- Help me i dont know how to do itarrow_forwardCan you explain how to draw a molecular orbital diagram for the given molecule? It is quite difficult to understand. Additionally, could you provide a clearer illustration? Furthermore, please explain how to draw molecular orbital diagrams for any other given molecule or compound as well.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Prob 10: Select to Add Arrows THEarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
