
Chemistry for Today: General Organic and Biochemistry
9th Edition
ISBN: 9781337514576
Author: Seager
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.14E
Classify the following alcohols as primary, secondary, or tertiary:
a.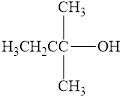
b.
c.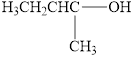
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Let's see if you caught the essentials of the animation.
What is the valence value of carbon?
a) 4
b) 2
c) 8
d) 6
A laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).
A laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).
Chapter 13 Solutions
Chemistry for Today: General Organic and Biochemistry
Ch. 13 - Draw general formulas for an alcohol and phenol,...Ch. 13 - Prob. 13.2ECh. 13 - Assign IUPAC names to the following alcohols: a....Ch. 13 - Assign IUPAC names to the following alcohols: a....Ch. 13 - Several important alcohols are well known by...Ch. 13 - Prob. 13.6ECh. 13 - Draw structural formulas for each of the...Ch. 13 - Draw structural formulas for each of the...Ch. 13 - Name each of the following as a derivative of...Ch. 13 - Name each of the following as a derivative of...
Ch. 13 - Prob. 13.11ECh. 13 - Draw structural formulas for each of the...Ch. 13 - Prob. 13.13ECh. 13 - Classify the following alcohols as primary,...Ch. 13 - Classify the following alcohols as primary,...Ch. 13 - Draw structural formulas for the four aliphatic...Ch. 13 - Why are the boiling points of alcohols much higher...Ch. 13 - Arrange the compounds of each group in order of...Ch. 13 - Prob. 13.19ECh. 13 - Draw structural formulas for the following...Ch. 13 - Prob. 13.21ECh. 13 - Draw the structures of the chief product formed...Ch. 13 - Draw the structures of the chief product formed...Ch. 13 - Draw the structures of the ethers that can be...Ch. 13 - Prob. 13.25ECh. 13 - Give the structure of an alcohol that could be...Ch. 13 - Give the structure of an alcohol that could be...Ch. 13 - What products would result from the following...Ch. 13 - What products would result from the following...Ch. 13 - Each of the following conversions requires more...Ch. 13 - Each of the following conversions requires more...Ch. 13 - The three-carbon diol used in antifreeze is It is...Ch. 13 - Methanol is fairly volatile and evaporates quickly...Ch. 13 - Prob. 13.34ECh. 13 - Prob. 13.35ECh. 13 - Name an alcohol used in each of the following...Ch. 13 - Prob. 13.37ECh. 13 - Prob. 13.38ECh. 13 - Assign a common name to each of the following...Ch. 13 - Assign a common name to each of the following...Ch. 13 - Assign the IUPAC name to each of the following...Ch. 13 - Assign the IUPAC name to each of the following...Ch. 13 - Prob. 13.43ECh. 13 - Draw structural formulas for the following: a....Ch. 13 - Prob. 13.45ECh. 13 - Prob. 13.46ECh. 13 - Prob. 13.47ECh. 13 - Arrange the following compounds in order of...Ch. 13 - Arrange the compounds in Exercise 13.48 in order...Ch. 13 - Prob. 13.50ECh. 13 - Complete the following reactions: a. b....Ch. 13 - Prob. 13.52ECh. 13 - Lipoic acid is required by many microorganisms for...Ch. 13 - Alcohols and thiols can both be oxidized in a...Ch. 13 - Prob. 13.55ECh. 13 - Prob. 13.56ECh. 13 - Prob. 13.57ECh. 13 - Thiols have lower boiling points and are less...Ch. 13 - Prob. 13.59ECh. 13 - Prob. 13.60ECh. 13 - Prob. 13.61ECh. 13 - Prob. 13.62ECh. 13 - A mixture of ethanol and 1propanol is heated to...Ch. 13 - Prob. 13.64ECh. 13 - Prob. 13.65ECh. 13 - Prob. 13.66ECh. 13 - Prob. 13.67ECh. 13 - Figure 13.8 points out that methanol is used as a...Ch. 13 - Figure 13.13 focuses on the use of thiol chemistry...Ch. 13 - Prob. 13.70ECh. 13 - Prob. 13.71ECh. 13 - Prob. 13.72ECh. 13 - The compound that has the greatest polarity is: a....Ch. 13 - Alcoholic beverages contain: a. wood alcohol. b....Ch. 13 - Prob. 13.75ECh. 13 - Which of the following compounds is an ether? a....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The number of microstates corresponding to each macrostate is given by N. The dominant macrostate or configuration of a system is the macrostate with the greatest weight W. Are both statements correct?arrow_forwardFor the single step reaction: A + B → 2C + 25 kJ If the activation energy for this reaction is 35.8 kJ, sketch an energy vs. reaction coordinate diagram for this reaction. Be sure to label the following on your diagram: each of the axes, reactant compounds and product compounds, enthalpy of reaction, activation energy of the forward reaction with the correct value, activation energy of the backwards reaction with the correct value and the transition state. In the same sketch you drew, after the addition of a homogeneous catalyst, show how it would change the graph. Label any new line "catalyst" and label any new activation energy.arrow_forwardHow many grams of C are combined with 3.75 ✕ 1023 atoms of H in the compound C5H12?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License