
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.SE, Problem 48AP
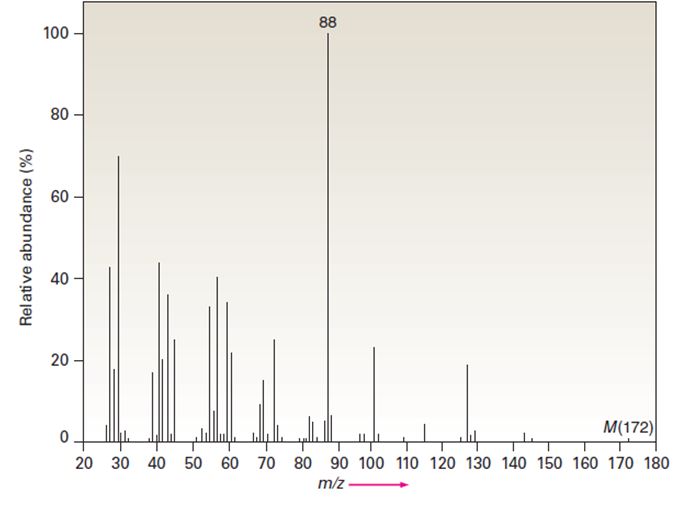
The infrared spectrum of the compound with the mass spectrum shown below lacks any significant absorption above 3000 cm-1. There is a prominent peak near 1740 cm-1 and another strong peak near 1200 cm-1. Propose a structure consistent with the data.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
None
Redraw the molecule below as a skeletal ("line") structure. Be sure to use wedge and dash bonds if necessary to accurately
represent the direction of the bonds to ring substituents.
Cl.
Br
Click and drag to start drawing a
structure.
: ☐
☑
P
K
m
Choose the best reagents to complete the following reaction.
L
ZI
0
Problem 4 of 11
A
1. NaOH
2. CH3CH2CH2NH2
1. HCI
B
OH
2. CH3CH2CH2NH2
DII
F1
F2
F3
F4
F5
A
F6
C
CH3CH2CH2NH2
1. SOCl2
D
2. CH3CH2CH2NH2
1. CH3CH2CH2NH2
E
2. SOCl2
Done
PrtScn
Home
End
FA
FQ
510
*
PgUp
M
Submit
PgDn
F11
Chapter 12 Solutions
Organic Chemistry
Ch. 12.2 - Prob. 1PCh. 12.2 - Two mass spectra are shown in FIGURE 12-8. One...Ch. 12.3 - What are the masses of the charged fragments...Ch. 12.3 - Prob. 4PCh. 12.5 - Prob. 5PCh. 12.5 - Prob. 6PCh. 12.7 - What functional groups might the following...Ch. 12.7 - How might you use IR spectroscopy to distinguish...Ch. 12.8 - Prob. 9PCh. 12.8 - Where might the following compounds have IR...
Ch. 12.8 - Where might the following compound have IR...Ch. 12.SE - Prob. 12VCCh. 12.SE - Show the structures of the fragments you would...Ch. 12.SE - Propose structures for compounds that fit the...Ch. 12.SE - Write molecular formulas for compounds that show...Ch. 12.SE - Camphor, a saturated monoketone from the Asian...Ch. 12.SE - The nitrogen rule of mass spectrometry says that a...Ch. 12.SE - In light of the nitrogen rule mentioned in Problem...Ch. 12.SE - Nicotine is a diamino compound isolated from dried...Ch. 12.SE - The hormone cortisone contains C, H, and O, and...Ch. 12.SE - Halogenated compounds are particularly easy to...Ch. 12.SE - Prob. 22APCh. 12.SE - Propose structures for compounds that fit the...Ch. 12.SE - 2-Methylpentane (C6H14) has the mass spectrum...Ch. 12.SE - Assume that you are in a laboratory carrying out...Ch. 12.SE - What fragments might you expect in the mass...Ch. 12.SE - How might you use IR spectroscopy to distinguish...Ch. 12.SE - Would you expect two enantiomers such as...Ch. 12.SE - Would you expect two diastereomers such as meso-2,...Ch. 12.SE - Propose structures for compounds that meet the...Ch. 12.SE - How could you use infrared spectroscopy to...Ch. 12.SE - Prob. 32APCh. 12.SE - At what approximate positions might the following...Ch. 12.SE - How would you use infrared spectroscopy to...Ch. 12.SE - At what approximate positions might the following...Ch. 12.SE - Assume that you are carrying out the dehydration...Ch. 12.SE - Assume that you are carrying out the base-induced...Ch. 12.SE - Prob. 38APCh. 12.SE - Carvone is an unsaturated ketone responsible for...Ch. 12.SE - Prob. 40APCh. 12.SE - The mass spectrum (a) and the infrared spectrum...Ch. 12.SE - The mass spectrum (a) and the infrared spectrum...Ch. 12.SE - Propose structures for compounds that meet the...Ch. 12.SE - 4-Methyl-2-pentanone and 3-methylpentanal are...Ch. 12.SE - Grignard reagents undergo a general and very...Ch. 12.SE - Ketones undergo a reduction when treated with...Ch. 12.SE - Nitriles, R–=C≡N, undergo a hydrolysis...Ch. 12.SE - The infrared spectrum of the compound with the...Ch. 12.SE - The infrared spectrum of the compound with the...Ch. 12.SE - Prob. 50AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- given cler asnwerarrow_forwardAdd curved arrows to the reactants in this reaction. A double-barbed curved arrow is used to represent the movement of a pair of electrons. Draw curved arrows. : 0: si H : OH :: H―0: Harrow_forwardConsider this step in a radical reaction: Br N O hv What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. O primary Otermination O initialization O electrophilic O none of the above × ☑arrow_forward
- Nonearrow_forwardCan I get a drawing of what is happening with the orbitals (particularly the p orbital) on the O in the OH group? Is the p orbital on the O involved in the ring resonance? Why or why not?arrow_forward1) How many monochlorination products-including stereochemistry- are there for the molecule below:arrow_forward
- Select an amino acid that has and N-H or O-H bond in its R-group (you have 8 to choose from!). Draw at least two water molecules interacting with the R-group of the amino acid.arrow_forwardIs this aromatic?arrow_forwardCHEM2323 E Tt PS CH03 Draw and name all monobromo derivatives of pentane, C5H11Br. Problem 3-33 Name: Draw structures for the following: (a) 2-Methylheptane (d) 2,4,4-Trimethylheptane Problem 3-35 (b) 4-Ethyl-2,2-dimethylhexane (e) 3,3-Diethyl-2,5-dimethylnonane (c) 4-Ethyl-3,4-dimethyloctane 2 (f) 4-Isopropyl-3-methylheptane KNIE>arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY