
EBK ORGANIC CHEMISTRY-STUD.SOLNS.MAN+SG
4th Edition
ISBN: 9781119659525
Author: Klein
Publisher: WILEY CONS
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 12, Problem 69IP
Interpretation Introduction
Interpretation:
A proper mechanism for the following transformation is to be determined.
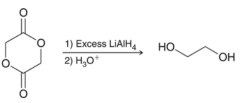
Concept introduction:
Hydride transfer is the migration of a hydrogen nucleus from one atom to another atom, accompanied by two electrons. The
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
H
Draw the product and the mechanism
1.) LDA
+
2.) H30*
H
○ excess
OH
Given the first-order reaction: aA → products. State its kinetic equation.
Draw the product and the mechanism
excess
A.
ob
B.
C.
H*;
.OH
OH
H*:
✓ OH
H*; H₂O
D.
excess
8
1. LDA
2. H*
Chapter 12 Solutions
EBK ORGANIC CHEMISTRY-STUD.SOLNS.MAN+SG
Ch. 12.1 - Prob. 1LTSCh. 12.1 - Prob. 1PTSCh. 12.1 - Prob. 2ATSCh. 12.1 - Prob. 3CCCh. 12.2 - Prob. 4CCCh. 12.3 - Prob. 8CCCh. 12.3 - Prob. 9CCCh. 12.4 - Prob. 3LTSCh. 12.4 - Prob. 10PTSCh. 12.4 - Prob. 11ATS
Ch. 12.4 - Prob. 4LTSCh. 12.4 - Prob. 12PTSCh. 12.4 - Prob. 13ATSCh. 12.6 - Prob. 14CCCh. 12.6 - Prob. 5LTSCh. 12.6 - Prob. 15PTSCh. 12.6 - Prob. 16PTSCh. 12.7 - Prob. 18CCCh. 12.9 - Prob. 6LTSCh. 12.9 - Prob. 19PTSCh. 12.9 - Prob. 20ATSCh. 12.9 - Prob. 21CCCh. 12.10 - Prob. 7LTSCh. 12.10 - Prob. 22PTSCh. 12.10 - Prob. 23ATSCh. 12.13 - Prob. 8LTSCh. 12.13 - Prob. 24PTSCh. 12.13 - Prob. 25ATSCh. 12.13 - Prob. 26CCCh. 12.13 - Prob. 9LTSCh. 12.13 - Prob. 27PTSCh. 12.13 - Prob. 28ATSCh. 12 - Prob. 29PPCh. 12 - Prob. 30PPCh. 12 - Prob. 31PPCh. 12 - Prob. 32PPCh. 12 - Prob. 33PPCh. 12 - Prob. 34PPCh. 12 - Prob. 35PPCh. 12 - Prob. 36PPCh. 12 - Prob. 37PPCh. 12 - Prob. 38PPCh. 12 - Prob. 39PPCh. 12 - Prob. 40PPCh. 12 - Prob. 41PPCh. 12 - Prob. 42PPCh. 12 - Prob. 43PPCh. 12 - Prob. 44PPCh. 12 - Prob. 45PPCh. 12 - Prob. 46PPCh. 12 - Prob. 47PPCh. 12 - Prob. 48PPCh. 12 - Prob. 49PPCh. 12 - Prob. 50PPCh. 12 - Prob. 51PPCh. 12 - Prob. 52PPCh. 12 - Prob. 53ASPCh. 12 - Prob. 54ASPCh. 12 - Prob. 55ASPCh. 12 - Prob. 62IPCh. 12 - Prob. 64IPCh. 12 - Prob. 65IPCh. 12 - Prob. 66IPCh. 12 - Prob. 67IPCh. 12 - Prob. 68IPCh. 12 - Prob. 69IPCh. 12 - Prob. 74IP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forwardPredict the product of the following reactions: O 0= excess Х Кон ОН H+ H+ Iarrow_forwardHow many chiral centers/stereocenters are there in the following molecule? 1 2 3 4arrow_forward
- Which of these correspond to the molecule: 2,5-dimethylheptanearrow_forwardGiven the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forwardWhich one of the following molecules is chiral? H- NH₂ H3C དང་།་ OH H HO H₂N HO- -H CHO -OH H HO- OH H- -H CH₂OH OHarrow_forward
- The structure of an unsaturated phospholipid is shown below. Which region of the molecule is most hydrophilic ? H₂N-CH₂ H₂C IV CH3 CH3 hydro-water philic-likes = Hydrophilic likes water ○ IV All regions are equally hydrophilic. IIIarrow_forwardWhich of the following compounds would you most appropriately call hydrophobic? ○ CH4 H2CO CO HCI ○ NaClarrow_forwardWhich of the following triglycerides would you most expect to be a liquid at room temperature? saturated fat trans monounsaturated fat trans polyunsaturated fat cis monounsaturated fat ○ cis polyunsaturated fatarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License