
Concept explainers
(a)
Interpretation:
The molecular formula for the given cycloalkane structure has to be found.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and
Cycloalkanes are a class of saturated hydrocarbons that contain a ring of carbon atoms with or without alkyl substituents on it. The general molecular formula for cycloalkanes is
In a line-angle structural formula of cycloalkanes, the intersection of two lines represent a methylene group (
(a)
Answer to Problem 12.95EP
The molecular formula for the given cycloalkane is
Explanation of Solution
Given cycloalkane is,

The above structure is a line-angle structural formula representation. This structure has a total of four intersections. Therefore, the total number of carbon atoms present in the given structure is found to be four.
The molecular formula for the given cycloalkane can be found using the general molecular formula. General molecular formula for cycloalkane is
The molecular formula for the given cycloalkane is found to be
The molecular formula for the given cycloalkane is found out using the general molecular formula for cycloalkanes.
(b)
Interpretation:
The molecular formula for the given cycloalkane structure has to be found.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Cycloalkanes are a class of saturated hydrocarbons that contain a ring of carbon atoms with or without alkyl substituents on it. The general molecular formula for cycloalkanes is
In a line-angle structural formula of cycloalkanes, the intersection of two lines represent a methylene group (
(b)
Answer to Problem 12.95EP
The molecular formula for the given cycloalkane is
Explanation of Solution
Given cycloalkane is,
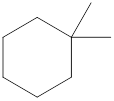
The above structure is a line-angle structural formula representation. This structure has a total of six intersections and two end lines. Therefore, the total number of carbon atoms present in the given structure is found to be eight.
The molecular formula for the given cycloalkane can be found using the general molecular formula. General molecular formula for cycloalkane is
The molecular formula for the given cycloalkane is found to be
The molecular formula for the given cycloalkane is found out using the general molecular formula for cycloalkanes.
(c)
Interpretation:
The molecular formula for the given cycloalkane structure has to be found.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Cycloalkanes are a class of saturated hydrocarbons that contain a ring of carbon atoms with or without alkyl substituents on it. The general molecular formula for cycloalkanes is
In a line-angle structural formula of cycloalkanes, the intersection of two lines represent a methylene group (
(c)
Answer to Problem 12.95EP
The molecular formula for the given cycloalkane is
Explanation of Solution
Given cycloalkane is,
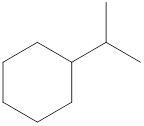
The above structure is a line-angle structural formula representation. This structure has a total of seven intersections and two end lines. Therefore, the total number of carbon atoms present in the given structure is found to be nine.
The molecular formula for the given cycloalkane can be found using the general molecular formula. General molecular formula for cycloalkane is
The molecular formula for the given cycloalkane is found to be
The molecular formula for the given cycloalkane is found out using the general molecular formula for cycloalkanes.
(d)
Interpretation:
The molecular formula for the given cycloalkane structure has to be found.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Cycloalkanes are a class of saturated hydrocarbons that contain a ring of carbon atoms with or without alkyl substituents on it. The general molecular formula for cycloalkanes is
In a line-angle structural formula of cycloalkanes, the intersection of two lines represent a methylene group (
(d)
Answer to Problem 12.95EP
The molecular formula for the given cycloalkane is
Explanation of Solution
Given cycloalkane is,
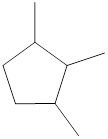
The above structure is a line-angle structural formula representation. This structure has a total of five intersections and three end lines. Therefore, the total number of carbon atoms present in the given structure is found to be eight.
The molecular formula for the given cycloalkane can be found using the general molecular formula. General molecular formula for cycloalkane is
The molecular formula for the given cycloalkane is found to be
The molecular formula for the given cycloalkane is found out using the general molecular formula for cycloalkanes.
Want to see more full solutions like this?
Chapter 12 Solutions
GENERAL,ORGANIC,+BIO.CHEM.-MINDTAP
- Determine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forwardDescribe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forwardState two similarities between fluorescence and phosphorescence.arrow_forward
- State three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forwardIn a photochemical reaction, how is the rate of the process related to its quantum yield?arrow_forwardPrimary and global quantum yields in photochemistry. Define them and give their formulas. Differentiate between them.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





