
Concept explainers
Sharpless
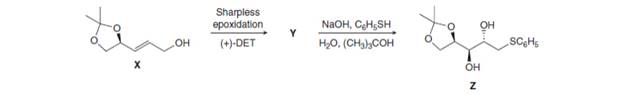
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Additional Science Textbook Solutions
Principles of Chemistry: A Molecular Approach (3rd Edition)
Organic Chemistry
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Chemistry: Structure and Properties
- One compound that contributes to the “seashore smell” at beaches in Hawai‘i is dictyopterene D', a component of a brown edible seaweed called limu lipoa. Hydrogenation of dictyopterene D' with excess H2 in the presence of a Pd catalyst forms butylcycloheptane. Ozonolysis with O3 followed by (CH3)2S forms CH2(CHO)2, HCOCH2CH(CHO)2, and CH3CH2CHO. What are possible structures of dictyopterene D'?arrow_forwardA 2-bromobutane react with methanol and form a enantiomeric pair of 2-methoxybutane. Draw the structures of the enntiomeric pairs of ethers.arrow_forwardButanol is treated with H2SO4 and heat. Identify the reaction and give the product that can be formed from itarrow_forward
- (a) Draw the products (including stereoisomers) formed when 2methylhex-2-ene is treated with HBr in the presence of peroxides. (b) Draw the products (including stereoisomers) formed when (S)-2,4dimethylhex-2-ene is treated with HBr and peroxides under similar conditions.arrow_forward34. Compound Z has the molecular formula CaHeO2. When Z is treated with LIAIH, in ether followed by Hz20/H*, the organic compound 2-phenylethanol is formed. Draw the structure of Z.arrow_forwardA synthetic organic molecule, G, which contains both aldehyde and ether functional groups, is subjected to a series of reactions in a multi-step synthesis pathway. In the first step, G undergoes a Wittig reaction, leading to the formation of an alkene, H. Subsequently, H is treated with an ozone (O3) reagent followed by a reducing agent in an ozonolysis reaction, resulting in the formation of two different products, I and J. Considering the functional groups present in G and the nature of the reactions involved, what are the most probable structures or functional groups present in products I and J? A. I contains a carboxylic acid group, and J contains an aldehyde group. B. I contains a ketone group, and J contains an alcohol group. C. I and J both contain aldehyde groups. D. I contains an ester group, and J contains a ketone group. Don't use chat gpt.arrow_forward
- Q6arrow_forwardChrysanthemic acid occurs as a mixture of esters in flowers of the chrysanthemum (pyrethrum) family. Reduction of chrysanthemic acid to its alcohol followed by conversion of the alcohol to its tosylate gives chrysanthemyl tosylate. Solvolysis alcohols. of the tosylate gives a mixture of artemesia and yomogi HO НО Chrysanthemic acid Chrysanthemyl alcohol OH TsO DMSO Chrysanthemyl tosylate Artemesia alcohol Yomogi alcohol Propose a mechanism for the formation of these alcohols from chrysanthemyl tosylate.arrow_forwardAn alkene is treated with OsO4 followed by H2O2. When the resulting diol is treated with HIO4, the only product obtained is an unsubstituted cyclic ketone with molecular formula C6H10O. What is the structure of the alkene?arrow_forward
- 5. Fill in the missing reagent or the product: (b) (a) (c) RCO,H N(CH3)2 H₂O* (d)arrow_forwardIn addition to using CHX3 and base to synthesize dihalocarbenes (Section 26.4), dichlorocarbene (:CCl2) can be prepared by heating sodium trichloroacetate. Draw a stepwise mechanism for this reaction.arrow_forwardQuinapril (trade name Accupril) is used to treat high blood pressure andcongestive heart failure. One step in the synthesis of quinapril involvesreaction of the racemic alkyl bromide A with a single enantiomer of theamino ester B. Given the structure of quinapril, which one of these two products isneeded to synthesize the drug?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

