
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
5th Edition
ISBN: 9781260170405
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.69P
Devise a synthesis of each compound from
a. 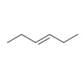 b.
b. 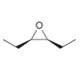 c.
c. 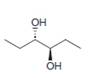
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Li2CrO4
is this Lithium (II) Chromate
Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under
the table.
NH
**
Molecule 1
NH
Molecule 4
none of the above
Х
Molecule 3
Molecule 2
H
N
wwwwww..
HN
Molecule 5
Molecule 6
HN
R
mw...
N
H
☐
Nomenclature P4S3
Would this be tetraphsophorus tri sulfide?
Chapter 12 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Ch. 12 - Prob. 12.1PCh. 12 - Problem 12.2 What alkane is formed when each...Ch. 12 - Prob. 12.3PCh. 12 - Prob. 12.4PCh. 12 - Prob. 12.5PCh. 12 - Prob. 12.6PCh. 12 - Compound Molecular formula before...Ch. 12 - Problem 12.8 Draw the products formed when...Ch. 12 - Prob. 12.9PCh. 12 - Prob. 12.10P
Ch. 12 - Problem 12.11 (a) Draw the structure of a compound...Ch. 12 - Prob. 12.12PCh. 12 - Prob. 12.13PCh. 12 - Problem 12.14 Draw the products of each...Ch. 12 - Prob. 12.15PCh. 12 - Problem 12.16 Draw all stereoisomers formed when...Ch. 12 - Prob. 12.17PCh. 12 - Problem 12.18 Draw the products formed when both...Ch. 12 - Problem 12.19 Draw the products formed when each...Ch. 12 - Prob. 12.20PCh. 12 - Prob. 12.21PCh. 12 - Problem 12.22 Draw the products formed when each...Ch. 12 - Prob. 12.23PCh. 12 - Problem 12.24 Draw the organic products in each of...Ch. 12 - Prob. 12.25PCh. 12 - Prob. 12.26PCh. 12 - Problem 12.27 Draw the products of each Sharpless...Ch. 12 - Prob. 12.28PCh. 12 - 12.29 Draw the products formed when A is treated...Ch. 12 - Prob. 12.30PCh. 12 - 12.31 Devise a synthesis of the following compound...Ch. 12 - Prob. 12.32PCh. 12 - Prob. 12.33PCh. 12 - Prob. 12.34PCh. 12 - Prob. 12.35PCh. 12 - Prob. 12.36PCh. 12 - 12.37 Stearidonic acid (C18H28O2) is an...Ch. 12 - Draw the organic products formed when cyclopentene...Ch. 12 - Draw the organic products formed when allylic...Ch. 12 - Draw the organic products formed in each reaction....Ch. 12 - Prob. 12.41PCh. 12 - Prob. 12.42PCh. 12 - Prob. 12.43PCh. 12 - What alkene is needed to synthesize each 1,2-diol...Ch. 12 - Prob. 12.45PCh. 12 - 12.46 (a)What product is formed in Step [1] of the...Ch. 12 - Draw the products formed after Steps 1 and 2 in...Ch. 12 - 12.48 Draw the products formed in each oxidative...Ch. 12 - What alkene or alkyne yields each set of products...Ch. 12 - Prob. 12.50PCh. 12 - Prob. 12.51PCh. 12 - Prob. 12.52PCh. 12 - Prob. 12.53PCh. 12 - 12.54 An unknown compound A of molecular formula ...Ch. 12 - 12.55 DHA is a fatty acid derived from fish oil...Ch. 12 - Prob. 12.56PCh. 12 - 12.57 Draw the product of each asymmetric...Ch. 12 - 12.58 Epoxidation of the following allylic alcohol...Ch. 12 - Prob. 12.59PCh. 12 - 12.60 Identify A in the following reaction...Ch. 12 - Prob. 12.61PCh. 12 - 12.62 It is sometimes necessary to isomerize a cis...Ch. 12 - 12.63 Devise a synthesis of each compound from...Ch. 12 - Prob. 12.64PCh. 12 - Prob. 12.65PCh. 12 - 12.66 Devise a synthesis of each compound from the...Ch. 12 - Prob. 12.67PCh. 12 - Prob. 12.68PCh. 12 - 12.69 Devise a synthesis of each compound from as...Ch. 12 - Prob. 12.70PCh. 12 - Prob. 12.71PCh. 12 - 12.72 Draw a stepwise mechanism for the following...Ch. 12 - Prob. 12.73PCh. 12 - Prob. 12.74PCh. 12 - 12.75 Sharpless epoxidation of allylic alcohol X...
Additional Science Textbook Solutions
Find more solutions based on key concepts
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
1. Rub your hands together vigorously. What happens? Discuss the energy transfers and transformations that take...
College Physics: A Strategic Approach (3rd Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used Ai solutionarrow_forwardBenzene-toluene equilibrium is often approximated as αBT = 2.34. Generate the y-x diagram for this relative volatility. Also, generate the equilibrium data using Raoult’s law, and compare your results to these.arrow_forwardGiven the most probable macrostate: s/k (K) Populations 300 4 200 8 100 16 0 32 Indicate how to demonstrate that the population of the levels is consistent with the Boltzmann distribution.arrow_forward
- Rank the following components in order of decreasing volatility: butane, n-pentane, iso-pentene (e.g., 3-methyl-1-butene), isoprene, pentanol? Briefly explain your answer.arrow_forwardViscosity of a liquid related to the activation energy.arrow_forwardVibrational contributions to internal energy and heat capacity1) are temperature independent2) are temperature dependentarrow_forward
- The approximation of calculating the partition function by integration instead of the summation of all the energy terms can only be done if the separation of the energy levels is much smaller than the product kT. Explain why.arrow_forwardExplain the meaning of: the electron partition function is equal to the degeneracy of the ground state.arrow_forward28. For each of the following species, add charges wherever required to give a complete, correct Lewis structure. All bonds and nonbonded valence electrons are shown. a. b. H H H H H :0-C-H H H H-C-H C. H H d. H-N-0: e. H H-O H-O H B=0 f. H—Ö—Ñ—Ö—H Norton Private Barrow_forward
- At 0oC and 1 atm, the viscosity of hydrogen (gas) is 8.55x10-5 P. Calculate the viscosity of a gas, if possible, consisting of deuterium. Assume that the molecular sizes are equal.arrow_forwardIndicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardIndicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY