
Concept explainers
(a)
Interpretation: The number of rings and number of pi bonds in A is to be determined. And one possible structure is to be drawn.
Concept introduction: Degree of unsaturation is used to determine the total number of rings and pi bonds present in compound by just looking at the molecular formula. It does not specify the total number of rings and total number of pi bonds individually.
Answer to Problem 12.35P
The number of pi bonds and number of rings in A is one. The possible structure is given in Figure 1.
Explanation of Solution
For compound A:
Before hydrogenation, the molecular formula is
The maximum number of
The maximum number of
The number of
Substitute the values of maximum number of
The degree of unsaturation is calculated by the formula,
Hence, the degree of unsaturation before hydrogenation is two.
After hydrogenation, the molecular formula is
The maximum number of
The maximum number of
The number
Substitute the values of maximum number of
The degree of unsaturation is calculated by the formula,
Hence, the degree of unsaturation after hydrogenation is one.
The number of pi bonds in A is calculated by the formula,
Substitute the values of degree of unsaturation before hydrogenation and degree of unsaturation after hydrogenation in the above formula.
Hence, the number of pi bonds is one.
Number of rings is calculated by the formula,
Substitute the values of degree of unsaturation and number of pi bonds in the above formula.
Hence, the number of rings is one.
The possible structure for A is,
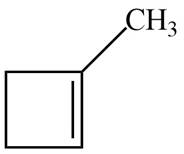
Figure 1
The number of pi bonds and number of rings in A is one. The possible structure is given in Figure 1.
(b)
Interpretation: The number of rings and number of pi bonds in B is to be determined. And one possible structure is to be drawn.
Concept introduction: Degree of unsaturation is used to determine the total number of rings and pi bonds present in compound by just looking at the molecular formula. It does not specify the total number of rings and total number of pi bonds individually.
Answer to Problem 12.35P
The number of pi bonds and number of rings in B is one and two respectively. The possible structure is given in Figure 2.
Explanation of Solution
For compound B:
Before hydrogenation, the molecular formula is
The maximum number of
The maximum number of
The number of
Substitute the values of maximum number of
The degree of unsaturation is calculated by the formula,
Hence, the degree of unsaturation before hydrogenation is three.
After hydrogenation, the molecular formula is
The maximum number of
The maximum number of
The number
Substitute the values of maximum number of
The degree of unsaturation is calculated by the formula,
Hence, the degree of unsaturation after hydrogenation is two.
The number of pi bonds in A is calculated by the formula,
Substitute the values of degree of unsaturation before hydrogenation and degree of unsaturation after hydrogenation in the above formula.
Hence, the number of pi bonds is one.
Number of rings is calculated by the formula,
Substitute the values of degree of unsaturation and number of pi bonds in the above formula.
Hence, the number of rings is two.
The possible structure for B is,
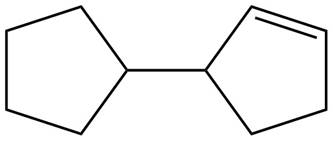
Figure 2
The number of pi bonds and number of rings in B is one and two respectively. The possible structure is given in Figure 2.
(c)
Interpretation: The number of rings and number of pi bonds in B is to be determined. And one possible structure is to be drawn.
Concept introduction: Degree of unsaturation is used to determine the total number of rings and pi bonds present in compound by just looking at the molecular formula. It does not specify the total number of rings and total number of pi bonds individually.
Answer to Problem 12.35P
The number of pi bonds and number of rings in C is four and one respectively. The possible structure is given in Figure 3.
Explanation of Solution
For compound C:
Before hydrogenation, the molecular formula is
The maximum number of
The maximum number of
The number of
Substitute the values of maximum number of
The degree of unsaturation is calculated by the formula,
Hence, the degree of unsaturation before hydrogenation is five.
After hydrogenation, the molecular formula is
The maximum number of
The maximum number of
The number
Substitute the values of maximum number of
The degree of unsaturation is calculated by the formula,
Hence, the degree of unsaturation after hydrogenation is one.
The number of pi bonds in A is calculated by the formula,
Substitute the values of degree of unsaturation before hydrogenation and degree of unsaturation after hydrogenation in the above formula.
Hence, the number of pi bonds is four.
Number of rings is calculated by the formula,
Substitute the values of degree of unsaturation and number of pi bonds in the above formula.
Hence, the number of rings is one.
The possible structure for C is,
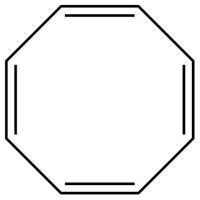
Figure 3
The number of pi bonds and number of rings in C is four and one respectively. The possible structure is given in Figure 3.
Want to see more full solutions like this?
Chapter 12 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- ↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forwardDon't used hand raitingarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Indicate the coordination forms of Si in silicates.arrow_forwardBriefly indicate the structure and bonding of silicates.arrow_forward4 Part C Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma.arrow_forward
- Try: Draw possible resonance contributing structures for the following organic species: CH3CH2NO2 [CH2CHCH2] [CH2CHCHO] [CH2CHCH2] [CH2CHNH2]arrow_forwardComplete the following synthesis. (d). H+ ง сarrow_forwardCan the target compound be efficiently synthesized in good yield from the substituted benzene of the starting material? If yes, draw the synthesis. Include all steps and all reactants.arrow_forward
