
Concept explainers
(a)
Interpretation:
The following pair of molecules should be classified as constitutional isomers or identical molecules or not isomers of each other:
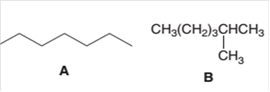
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds is
The molecular formula of an organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with
(b)
Interpretation:
The following pair of molecules should be classified as constitutional isomers or identical molecules or not isomers of each other:
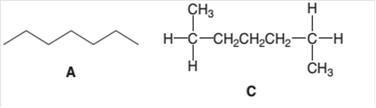
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds is organic chemistry.
The molecular formula of an organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule. Constitutional isomers can be defined as the pair of molecules with the same molecular formula but different structural formula.
(c)
Interpretation:
The following pair of molecules should be classified as constitutional isomers or identical molecules or not isomers of each other:
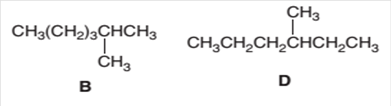
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds is organic chemistry.
The molecular formula of an organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule. Constitutional isomers can be defined as the pair of molecules with the same molecular formula but different structural formula.
(d)
Interpretation:
The following pair of molecules should be classified as constitutional isomers or identical molecules or not isomers of each other:
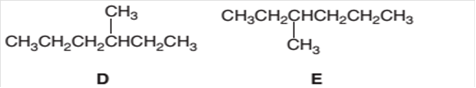
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds is organic chemistry.
The molecular formula of an organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule. Constitutional isomers can be defined as the pair of molecules with the same molecular formula but different structural formula.
(e)
Interpretation:
The following pair of molecules should be classified as constitutional isomers or identical molecules or not isomers of each other:
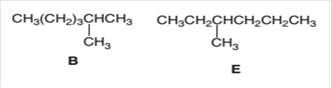
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds is organic chemistry.
The molecular formula of an organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule. Constitutional isomers can an as the pair of molecules with the same molecular formula but different structural formula.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- + C8H16O2 (Fatty acid) + 11 02 → 8 CO2 a. Which of the above are the reactants? b. Which of the above are the products? H2o CO₂ c. Which reactant is the electron donor? Futty acid d. Which reactant is the electron acceptor? e. Which of the product is now reduced? f. Which of the products is now oxidized? 02 #20 102 8 H₂O g. Where was the carbon initially in this chemical reaction and where is it now that it is finished? 2 h. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forward→ Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? d. Which reactants are the electron acceptors? e. Which of the products are now reduced? f. Which product is now oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. OCH 3 (Choose one) OH (Choose one) Br (Choose one) Explanation Check NO2 (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Aarrow_forward
- For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects O donating O withdrawing O no inductive effects Resonance Effects Overall Electron-Density ○ donating ○ withdrawing O no resonance effects O electron-rich O electron-deficient O similar to benzene Cl O donating O withdrawing ○ donating ○ withdrawing O no inductive effects O no resonance effects O Explanation Check O electron-rich O electron-deficient similar to benzene X © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forwardIdentifying electron-donating and For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects NH2 ○ donating NO2 Explanation Check withdrawing no inductive effects Resonance Effects Overall Electron-Density ○ donating O withdrawing O no resonance effects O donating O withdrawing O donating withdrawing O no inductive effects Ono resonance effects O electron-rich electron-deficient O similar to benzene O electron-rich O electron-deficient O similar to benzene olo 18 Ar 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check Х (Choose one) OH (Choose one) OCH3 (Choose one) OH (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- Assign R or S to all the chiral centers in each compound drawn below porat bg 9 Br Brarrow_forwarddescrive the energy levels of an atom and howan electron moces between themarrow_forwardRank each set of substituents using the Cahn-Ingold-Perlog sequence rules (priority) by numbering the highest priority substituent 1.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning  Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning





