
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
3rd Edition
ISBN: 9781259298424
Author: SMITH
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.63P
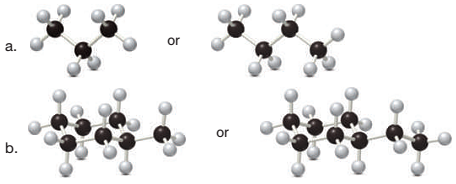
Which compound in each pair has the higher melting point?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Name the molecules & Identify any chiral center
CH3CH2CH2CHCH₂CH₂CH₂CH₂
OH
CH₂CHCH2CH3
Br
CH3
CH3CHCH2CHCH2CH3
CH3
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
Chapter 12 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
Ch. 12.1 - How many hydrogen atoms are present in each...Ch. 12.1 - Which formulas represent acyclic alkanes and which...Ch. 12.2 - Prob. 12.3PCh. 12.2 - Draw two isomers with molecular formula C6H14 that...Ch. 12.2 - Prob. 12.5PCh. 12.2 - Classify the carbon atoms in each compound as 1°,...Ch. 12.2 - Prob. 12.7PCh. 12.2 - Prob. 12.8PCh. 12.2 - Prob. 12.9PCh. 12.2 - Prob. 12.10P
Ch. 12.4 - Give the IUPAC name for each compound.Ch. 12.4 - Give the IUPAC name for each compound....Ch. 12.4 - Prob. 12.13PCh. 12.4 - Prob. 12.14PCh. 12.5 - Prob. 12.15PCh. 12.5 - Prob. 12.16PCh. 12.5 - Give the IUPAC name for each compound.Ch. 12.5 - Prob. 12.18PCh. 12.7 - Answer the following questions about pentane...Ch. 12.7 - Prob. 12.20PCh. 12.7 - Prob. 12.21PCh. 12.8 - Prob. 12.22PCh. 12.8 - Prob. 12.23PCh. 12.9 - Prob. 12.24PCh. 12.9 - Prob. 12.25PCh. 12.9 - Prob. 12.26PCh. 12 - Prob. 12.27PCh. 12 - Prob. 12.28PCh. 12 - Prob. 12.29PCh. 12 - Prob. 12.30PCh. 12 - Classify each carbon as 1°, 2°, 3°, or 4°. a....Ch. 12 - Prob. 12.32PCh. 12 - Label each pair of compounds as constitutional...Ch. 12 - Label each pair of compounds as constitutional...Ch. 12 - Consider compounds A, B, and C. Label each pair of...Ch. 12 - Consider compounds D,E, and F. Label each pair of...Ch. 12 - Prob. 12.37PCh. 12 - Prob. 12.38PCh. 12 - Draw structures that fit the following...Ch. 12 - Draw the five constitutional isomers having...Ch. 12 - Prob. 12.41PCh. 12 - Prob. 12.42PCh. 12 - Prob. 12.43PCh. 12 - Prob. 12.44PCh. 12 - Prob. 12.45PCh. 12 - Prob. 12.46PCh. 12 - Prob. 12.47PCh. 12 - Prob. 12.48PCh. 12 - Prob. 12.49PCh. 12 - Prob. 12.50PCh. 12 - Give the IUPAC name for each cycloalkane.Ch. 12 - Prob. 12.52PCh. 12 - Prob. 12.53PCh. 12 - Give the structure corresponding to each IUPAC...Ch. 12 - Each of the following IUPAC names is incorrect....Ch. 12 - Each of the following IUPAC names is incorrect....Ch. 12 - Draw three constitutional isomers having molecular...Ch. 12 - Draw four constitutional isomers having molecular...Ch. 12 - Draw a skeletal structure for each compound octane...Ch. 12 - Convert each compound to a skeletal structure CH3(...Ch. 12 - Convert each skeletal structure to a complete...Ch. 12 - Convert each skeletal structure to a complete...Ch. 12 - Which compound in each pair has the higher melting...Ch. 12 - Which compound in each pair has the higher boiling...Ch. 12 - Branching in an alkane chain decreases surface...Ch. 12 - Explain why the boiling points of heptane [CH3( CH...Ch. 12 - Explain why hexane is more soluble in...Ch. 12 - Mineral oil and Vaseline are both mixtures of...Ch. 12 - Write a balanced equation for the combustion of...Ch. 12 - Write a balanced equation for the combustion of...Ch. 12 - Write a balanced equation for the incomplete...Ch. 12 - Prob. 12.72PCh. 12 - Prob. 12.73PCh. 12 - Prob. 12.74PCh. 12 - Prob. 12.75PCh. 12 - Prob. 12.76PCh. 12 - Prob. 12.77PCh. 12 - Prob. 12.78PCh. 12 - Prob. 12.79PCh. 12 - Prob. 12.80PCh. 12 - Prob. 12.81PCh. 12 - Prob. 12.82PCh. 12 - Prob. 12.83PCh. 12 - A major component of animal fat is tristearin, (a)...Ch. 12 - Answer the following questions about the alkane...Ch. 12 - Prob. 12.86PCh. 12 - Prob. 12.87PCh. 12 - Answer the questions in Problem 12.85 for the...Ch. 12 - Prob. 12.89CPCh. 12 - Draw the structure of the 12 constitutional...Ch. 12 - Cyclopentane has a higher boiling point than...Ch. 12 - Prob. 12.92CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the IUPAC name of the following compound? CH₂CH₂ H CI H₂CH₂C H CH₂ Selected Answer: O (35,4R)-4 chloro-3-ethylpentane Correctarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forward
- Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardGiven 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY