
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
3rd Edition
ISBN: 9781259298424
Author: SMITH
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11.3, Problem 11.7P
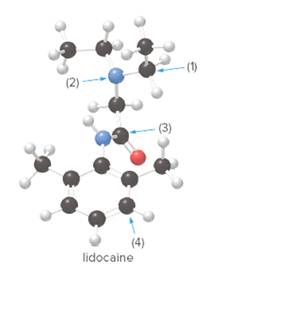
How many lone pairs are present in lidocaine, the drug mentioned in the chapter opener, whose structure is shown in the ball-and-stick model? Predict the shape around the indicated atoms in liodcaine.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Solve the spectro
Don't used hand raiting and don't used Ai solution
Don't used hand raiting and don't used Ai solution
Chapter 11 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
Ch. 11.1 - Prob. 11.1PCh. 11.2 - Fill in all H's and lone pairs in each compound.Ch. 11.3 - Prob. 11.3PCh. 11.3 - Prob. 11.4PCh. 11.3 - Prob. 11.5PCh. 11.3 - Prob. 11.6PCh. 11.3 - How many lone pairs are present in lidocaine, the...Ch. 11.4 - Convert each compound to a condensed formula.Ch. 11.4 - Convert each condensed formula to a complete...Ch. 11.4 - Convert each skeletal structure to a complete...
Ch. 11.4 - How many H’s are bonded to each indicated carbon...Ch. 11.5 - Prob. 11.12PCh. 11.5 - For each compound: [1] Identify the functional...Ch. 11.5 - Prob. 11.14PCh. 11.5 - Prob. 11.15PCh. 11.5 - Prob. 11.16PCh. 11.5 - Identify all of the functional groups in atenolol,...Ch. 11.5 - Prob. 11.18PCh. 11.5 - Prob. 11.19PCh. 11.6 - Indicate the polar bonds in each compound. Label...Ch. 11.6 - Prob. 11.21PCh. 11.6 - Prob. 11.22PCh. 11.6 - Predict the water solubility of each compound....Ch. 11.6 - Prob. 11.24PCh. 11.7 - Prob. 11.25PCh. 11.7 - Prob. 11.26PCh. 11 - Prob. 11.27PCh. 11 - Prob. 11.28PCh. 11 - Complete each structure by filling in all H’s and...Ch. 11 - Prob. 11.30PCh. 11 - Prob. 11.31PCh. 11 - Prob. 11.32PCh. 11 - Prob. 11.33PCh. 11 - Prob. 11.34PCh. 11 - “Ecstasy” is a widely used illegal stimulant....Ch. 11 - Prob. 11.36PCh. 11 - Explain why each C—C—C bond angle in benzene...Ch. 11 - Prob. 11.38PCh. 11 - Prob. 11.39PCh. 11 - Prob. 11.40PCh. 11 - Convert each compound to a skeletal structure.Ch. 11 - Convert each compound to a skeletal structure.Ch. 11 - Convert each shorthand structure to a complete...Ch. 11 - Convert each shorthand structure to a complete...Ch. 11 - A and B are ball-and-stick models of two compounds...Ch. 11 - Prob. 11.46PCh. 11 - What is wrong in each of the following shorthand...Ch. 11 - Prob. 11.48PCh. 11 - Prob. 11.49PCh. 11 - Albuterol (trade names Proventil and Ventolin) is...Ch. 11 - Prob. 11.51PCh. 11 - Prob. 11.52PCh. 11 - Prob. 11.53PCh. 11 - Prob. 11.54PCh. 11 - Prob. 11.55PCh. 11 - GHB is an addictive, illegal recreational drug...Ch. 11 - Prob. 11.57PCh. 11 - Prob. 11.58PCh. 11 - Prob. 11.59PCh. 11 - Prob. 11.60PCh. 11 - Prob. 11.61PCh. 11 - Prob. 11.62PCh. 11 - You are given two unlabeled bottles of solids, one...Ch. 11 - State how potassium iodide (KI) and pentane...Ch. 11 - The given beaker contains 100 mL of the organic...Ch. 11 - Prob. 11.66PCh. 11 - Why do we need to know the shape of a molecule...Ch. 11 - 1,1-Dichloroethylene (CH2=CCl2) is a starting...Ch. 11 - Indicate the polar bonds in each molecule. Label...Ch. 11 - Indicate the polar bonds in each molecule. Label...Ch. 11 - Classify each molecule as polar or nonpolar.Ch. 11 - Classify each molecule as polar or nonpolar. a....Ch. 11 - Which molecule is more water soluble? Explain.Ch. 11 - Explain why pantothenic acid, vitamin B5, is water...Ch. 11 - Prob. 11.75PCh. 11 - Prob. 11.76PCh. 11 - Explain why regularly taking a large excess of a...Ch. 11 - You can obtain the minimum daily requirement of...Ch. 11 - Prob. 11.79PCh. 11 - Vitamin B6 is obtained by eating a diet that...Ch. 11 - Prob. 11.81PCh. 11 - Can an oxygen-containing organic compound, have...Ch. 11 - Prob. 11.83PCh. 11 - Prob. 11.84PCh. 11 - Benzocaine is the active ingredient in topical...Ch. 11 - Methyl salicylate is responsible for the...Ch. 11 - Answer the following questions about aldosterone,...Ch. 11 - Answer the following questions about...Ch. 11 - Prob. 11.89PCh. 11 - Skin moisturizers come in two types, (a) One type...Ch. 11 - THC is the active component in marijuana (Section...Ch. 11 - Cocaine is a widely abused, addicting drug....
Additional Science Textbook Solutions
Find more solutions based on key concepts
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. 200 LOD For an unknown compound with a molecular ion of 101 m/z: a. Use the molecular ion to propose at least two molecular formulas. (show your work) b. What is the DU for each of your possible formulas? (show your work) C. Solve the structure and assign each of the following spectra. 8 6 4 2 (ppm) 150 100 50 ō (ppm) 4000 3000 2000 1500 1000 500 HAVENUMBERI-11arrow_forwardComplete the spectroscopy with structurearrow_forwardComplete the spectroscopy with structurearrow_forward
- Given the following concentrations for a system, calculate the value for the reaction quotient: Cl2(g)+ CS2(g) ⇌ CCl4(g)+ S2Cl2(g) Cl2 = 31.1 atm CS2 = 91.2 atm CCl4 = 2.12 atm S2Cl2 = 10.4 atmarrow_forwardMatch each chemical or item with the proper disposal or cleanup mwthod, Not all disposal and cleanup methods will be labeled. Metal sheets C, calcium, choroide solutions part A, damp metal pieces Part B, volumetric flask part A. a.Return to correct lables”drying out breaker. Place used items in the drawer.: Rinse with deionized water, dry as best you can, return to instructor. Return used material to the instructor.: Pour down the sink with planty of running water.: f.Pour into aqueous waste container. g.Places used items in garbage.arrow_forwardWrite the equilibrium constant expression for the following reaction: HNO2(aq) + H2O(l) ⇌ H3O+(aq) + NO2-(aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY