
Thermodynamics: An Engineering Approach
9th Edition
ISBN: 9781260048766
Author: CENGEL
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11.10, Problem 127RP
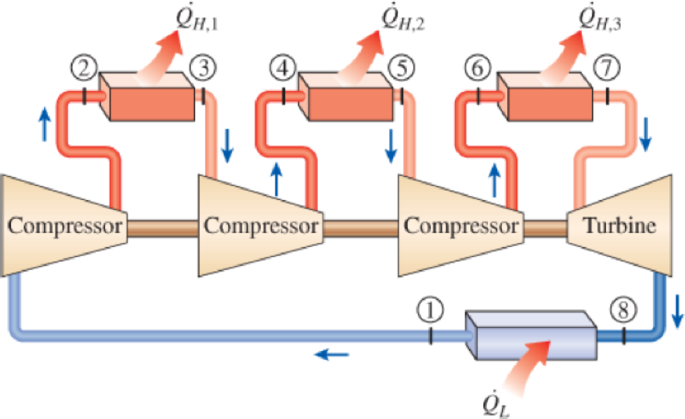
An ideal gas refrigeration system with three stages of compression with intercooling operates with air entering the first compressor at 50 kPa and −30°C. Each compressor in this system has a pressure ratio of 7, and the air temperature at the outlet of all intercoolers is 15°C. Calculate the COP of this system. Use constant specific heats at room temperature.
FIGURE P11–127
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A vapor compression refrigeration system with R-134a as refrigerant is used to cool down 0.1 m3/s air at 25°C and 20% RH to 15°C. The condenser outlet is at 36°C and 9.1 bar and the evaporator outlet is at 0°C and 2 bar. Calculate the power requirement of the compressor, COP of the system and the temperature of the refrigerant at compressor outlet.
show PH diagram and include also the solution of the interpolation
Avapour compression refrigeration system using refrigerant F-12 is employed to produce 8640 kg of ice per day. The condensing and evaporating temperature of refrigerant are 48°C and -20°C respectively. Saturated liquid leaves the condenser and saturated vapour leaves the evaporator. Compression is isentropic, water at 35 C is used to form the ice. The temperature of ice should be-8°C. Heat flow into the orme tank from surroundings may be taken as 10% of total heat absorbed from
water to form ice. Determine the power required to drive the compressor. Take specific heat of ice = 2,26 kJ/kg-K. Latent heat of Ice= 335 kJ/kg. Specific heat of water =
4.2 kJ/kg-K.
Chapter 11 Solutions
Thermodynamics: An Engineering Approach
Ch. 11.10 - Why do we study the reversed Carnot cycle even...Ch. 11.10 - Why is the reversed Carnot cycle executed within...Ch. 11.10 - A steady-flow Carnot refrigeration cycle uses...Ch. 11.10 - Refrigerant-134a enters the condenser of a...Ch. 11.10 - Does the ideal vapor-compression refrigeration...Ch. 11.10 - Why is the throttling valve not replaced by an...Ch. 11.10 - In a refrigeration system, would you recommend...Ch. 11.10 - Does the area enclosed by the cycle on a T-s...Ch. 11.10 - Consider two vapor-compression refrigeration...Ch. 11.10 - It is proposed to use water instead of...
Ch. 11.10 - The COP of vapor-compression refrigeration cycles...Ch. 11.10 - A 10-kW cooling load is to be served by operating...Ch. 11.10 - An ice-making machine operates on the ideal...Ch. 11.10 - An air conditioner using refrigerant-134a as the...Ch. 11.10 - An ideal vapor-compression refrigeration cycle...Ch. 11.10 - A refrigerator operates on the ideal...Ch. 11.10 - A refrigerator uses refrigerant-134a as the...Ch. 11.10 - An ideal vapor-compression refrigeration cycle...Ch. 11.10 - A refrigerator uses refrigerant-134a as its...Ch. 11.10 - A refrigerator uses refrigerant-134a as the...Ch. 11.10 - A commercial refrigerator with refrigerant-134a as...Ch. 11.10 - The manufacturer of an air conditioner claims a...Ch. 11.10 - Prob. 24PCh. 11.10 - How is the second-law efficiency of a refrigerator...Ch. 11.10 - Prob. 26PCh. 11.10 - Prob. 27PCh. 11.10 - Prob. 28PCh. 11.10 - Bananas are to be cooled from 28C to 12C at a rate...Ch. 11.10 - A vapor-compression refrigeration system absorbs...Ch. 11.10 - A room is kept at 5C by a vapor-compression...Ch. 11.10 - Prob. 32PCh. 11.10 - A refrigerator operating on the vapor-compression...Ch. 11.10 - When selecting a refrigerant for a certain...Ch. 11.10 - A refrigerant-134a refrigerator is to maintain the...Ch. 11.10 - Consider a refrigeration system using...Ch. 11.10 - A refrigerator that operates on the ideal...Ch. 11.10 - A heat pump that operates on the ideal...Ch. 11.10 - Do you think a heat pump system will be more...Ch. 11.10 - What is a water-source heat pump? How does the COP...Ch. 11.10 - A heat pump operates on the ideal...Ch. 11.10 - Refrigerant-134a enters the condenser of a...Ch. 11.10 - A heat pump that operates on the ideal...Ch. 11.10 - The liquid leaving the condenser of a 100,000...Ch. 11.10 - Reconsider Prob. 1144E. What is the effect on the...Ch. 11.10 - A heat pump using refrigerant-134a heats a house...Ch. 11.10 - A heat pump using refrigerant-134a as a...Ch. 11.10 - Reconsider Prob. 1148. What is the effect on the...Ch. 11.10 - Prob. 50PCh. 11.10 - How does the COP of a cascade refrigeration system...Ch. 11.10 - Consider a two-stage cascade refrigeration cycle...Ch. 11.10 - Can a vapor-compression refrigeration system with...Ch. 11.10 - Prob. 54PCh. 11.10 - A certain application requires maintaining the...Ch. 11.10 - Prob. 56PCh. 11.10 - Repeat Prob. 1156 for a flash chamber pressure of...Ch. 11.10 - Prob. 59PCh. 11.10 - A two-stage compression refrigeration system with...Ch. 11.10 - A two-stage compression refrigeration system with...Ch. 11.10 - A two-evaporator compression refrigeration system...Ch. 11.10 - A two-evaporator compression refrigeration system...Ch. 11.10 - Repeat Prob. 1163E if the 30 psia evaporator is to...Ch. 11.10 - Consider a two-stage cascade refrigeration cycle...Ch. 11.10 - How does the ideal gas refrigeration cycle differ...Ch. 11.10 - Prob. 67PCh. 11.10 - Devise a refrigeration cycle that works on the...Ch. 11.10 - How is the ideal gas refrigeration cycle modified...Ch. 11.10 - Prob. 70PCh. 11.10 - How do we achieve very low temperatures with gas...Ch. 11.10 - An ideal gas refrigeration system operates with...Ch. 11.10 - Air enters the compressor of an ideal gas...Ch. 11.10 - Repeat Prob. 1173 for a compressor isentropic...Ch. 11.10 - An ideal gas refrigeration cycle uses air as the...Ch. 11.10 - Rework Prob. 1176E when the compressor isentropic...Ch. 11.10 - A gas refrigeration cycle with a pressure ratio of...Ch. 11.10 - A gas refrigeration system using air as the...Ch. 11.10 - An ideal gas refrigeration system with two stages...Ch. 11.10 - Prob. 81PCh. 11.10 - Prob. 82PCh. 11.10 - What are the advantages and disadvantages of...Ch. 11.10 - Prob. 84PCh. 11.10 - Prob. 85PCh. 11.10 - Prob. 86PCh. 11.10 - Prob. 87PCh. 11.10 - Heat is supplied to an absorption refrigeration...Ch. 11.10 - An absorption refrigeration system that receives...Ch. 11.10 - An absorption refrigeration system receives heat...Ch. 11.10 - Heat is supplied to an absorption refrigeration...Ch. 11.10 - Prob. 92PCh. 11.10 - Prob. 93PCh. 11.10 - Consider a circular copper wire formed by...Ch. 11.10 - An iron wire and a constantan wire are formed into...Ch. 11.10 - Prob. 96PCh. 11.10 - Prob. 97PCh. 11.10 - Prob. 98PCh. 11.10 - Prob. 99PCh. 11.10 - Prob. 100PCh. 11.10 - Prob. 101PCh. 11.10 - Prob. 102PCh. 11.10 - A thermoelectric cooler has a COP of 0.18, and the...Ch. 11.10 - Prob. 104PCh. 11.10 - Prob. 105PCh. 11.10 - Prob. 106PCh. 11.10 - Rooms with floor areas of up to 15 m2 are cooled...Ch. 11.10 - Consider a steady-flow Carnot refrigeration cycle...Ch. 11.10 - Consider an ice-producing plant that operates on...Ch. 11.10 - A heat pump that operates on the ideal...Ch. 11.10 - A heat pump operates on the ideal...Ch. 11.10 - A large refrigeration plant is to be maintained at...Ch. 11.10 - Repeat Prob. 11112 assuming the compressor has an...Ch. 11.10 - An air conditioner with refrigerant-134a as the...Ch. 11.10 - A refrigerator using refrigerant-134a as the...Ch. 11.10 - Prob. 117RPCh. 11.10 - An air conditioner operates on the...Ch. 11.10 - Consider a two-stage compression refrigeration...Ch. 11.10 - A two-evaporator compression refrigeration system...Ch. 11.10 - The refrigeration system of Fig. P11122 is another...Ch. 11.10 - Repeat Prob. 11122 if the heat exchanger provides...Ch. 11.10 - An aircraft on the ground is to be cooled by a gas...Ch. 11.10 - Consider a regenerative gas refrigeration cycle...Ch. 11.10 - An ideal gas refrigeration system with three...Ch. 11.10 - Prob. 130RPCh. 11.10 - Derive a relation for the COP of the two-stage...Ch. 11.10 - Prob. 133FEPCh. 11.10 - Prob. 134FEPCh. 11.10 - Prob. 135FEPCh. 11.10 - Prob. 136FEPCh. 11.10 - Prob. 137FEPCh. 11.10 - An ideal vapor-compression refrigeration cycle...Ch. 11.10 - Prob. 139FEPCh. 11.10 - An ideal gas refrigeration cycle using air as the...Ch. 11.10 - Prob. 141FEPCh. 11.10 - Prob. 142FEP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A vapor-compression refrigeration system circulates refrigerant 134a at a rate of 0.15 kg/s. The refrigerant enters the compressor at -10 degrees Celcius and 100 kPa, and exits the compressor at 800 kPa. The isentropic efficiency of the compressor is 76%. Pressure drop through the condenser and evaporator are negligible. The refrigerant exits the condenser at 30 degrees Celcius and 800 kPa. Ignoring the heat transfer between the compressor and its surroundings, determine: The rate at which heat energy is removed from the refrigerated space in kW. The coefficient of perfromance.arrow_forwardSaturated vapor Freon 12 refrigerant at 219.12 kPa leaves the evaporator and enters the compressor at -5 °C. The refrigerant leaves the condenser as saturated liquid at 25°C and enters the ex pansion valve at 22°C. Heat rejected from the condenser amount to 75 kW. The work to the compressor is 55.5 kJ/kg, while the heat lost from the compressor is 4.2 kJ/kg. If 1.15 kJ/kg of heat are lost in the piping between the compressor and condenser, cal culate the refrigeration capacity in tons.arrow_forwardParrow_forward
- A vapor-compression system produces 20 tons of refrigeration using R12 as a refrigerant while operating between a condenser temperature of 41.6°c and an evaporator temperature of – 25°c. The superheat at the compression suction and the sub-cooling at the condenser outlet are 10°K. The pressure drop are 10kPa across the evaporator and the condenser. Determine the refrigerating effect in kJ per kilogram, the circulating rate of R12 in kilograms per second, the power required, the COP, the heat rejected in kW and the volume flow rate of refrigerant at compressor inlet conditions. 10° K subcooling 4 Tm= 41.6° C h= C s =C Tevap =-25° C 10° K superheatarrow_forwardAn ammonia vapor refrigeration cycle operates at an evaporator temperature of -16°C and a condensing temperature of 32°C. Determine the coefficient of performance for wet compression with superheated vapor leaving the compressor. The quality of vapor entering the compressor is 0.975 and the specific entropy of the superheated vapor discharging from the compressor is 5.7 kJ/kg-K.arrow_forwardA 100 x 300 mm ammonia compressor with a compression efficiency of 82% operates with a suction pressure of 291.57 kPa and a condenser pressure of 1203.7 kPa at 30 rps. The refrigerant cools 100 kg/min of water from 250C to 20C and assuming 5% of the useful refrigerating effect is lost due to the process of cooling, give that, h2 = 1653 kJ/kg, h1 = 1450.22 kJ/kg, h3 = 346.614 kJ/kg, v1 = 417.477 L/kg determine: a.) RE in tons of refrigeration b.) mass flow rate of ammonia c.) actual work of compression kW d.) find Brake work (kW) if mechanical eff is 90% e.) Actual volumetric eff f.) clearance volumetric eff if c=6%arrow_forward
- For a 10-ton-capacity refrigeration system, the pressure of refrigerant in the evaporator is 210 kPa, whereas in the condenser it is 800 kPa. If R-314a is used under saturated conditions, calculate the theoretical power required to operate the compressor.arrow_forwardA vapour compression refrigerator uses methyl chloride and works in the pressure range of 11.9 bar and5.67 bar. At the beginning of the compression, the refrigerant is 0.96 dry and at the end of isentropiccompression, it has a temperature of 55°C. The refrigerant liquid leaving the condenser is saturated. If themass flow of refrigerant is 1.8 kg/min. Determine :(i) Co-efficient of performance.(ii) The rise in temperature of condenser cooling water if the water flow rate is 16 kg/min.(iii) The ice produced in the evaporator in kg/hour from water at 15°C and ice at 0°C. Take : Specific enthalpy of fusion of ice = 336 kJ/kgSpecific heat of water = 4.187 kJ/kg.arrow_forwardRefrigerant-134a enters the compressor of a cooling system as superheated vapor at 0.18 MPa and 0°C with a flow rate of 0.15 kg/s. It exits the compressor at 0.8 MPa and 60°C. Post compression, the refrigerant is cooled in the condenser to 28°C and 0.7 MPa. Subsequently, it's throttled to 0.16 MPa. Neglecting any heat transfer and pressure drops in the pipelines between the components, represent the cycle on a T-s diagram concerning saturation lines. Calculate: (a) The rate of heat extraction from the cooling area and the energy input to the compressor. (b) The isentropic efficiency of the compressor. (c) The Coefficient of Performance (COP) of the cooling system.arrow_forward
- A 1200 kW water-cooled chiller has a centrifugal compressor with refrigerant R134a as the working fluid. The compressor runs at 9600 rev/min and has a slip factor of 0.95 and an overall isentropic efficiency of 90%. The impeller tip speed is not to exceed 160m/s. The evaporating and condensing temperatures are 2°C and 40°C respectively. Assuming that there is no superheating and sub-cooling, a) sketch the refrigration cycle on a P-h diagram (please indicate the pressures and specific enthalpies of all points in the cycle); b) determine the mass flow rate of the refrigerant R134a; c) determine the coefficient of performance of the chilled water plant; and discuss the possible measures to uplift the plant COP d) determine the number of stages and the impeller tip diameters, assuming that all stages would share same work input and each stage compression would have the same impeller tip diameter. e) Please also comment how the slip factor can affect the energy performance of the…arrow_forwardHello, please solve this problem, Thank you very mucharrow_forwardA 540,000 kJ/h capacity refrigeration system using Refrigerant 22 and served by a single compressor unit operates at an evaporator temperature of -23 oC and a condensing saturation temperature 40oC. To get rid of the single compressor load, a modification is proposed to use two stage of compression with refrigerant intercooling. (a) Determine the power required in the original system. (b) Determind the power required for the proposed modified system.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
The Refrigeration Cycle Explained - The Four Major Components; Author: HVAC Know It All;https://www.youtube.com/watch?v=zfciSvOZDUY;License: Standard YouTube License, CC-BY