
Concept explainers
The process that causes the greatest amount of exergy destruction.
Answer to Problem 117RP
The process that causes the greatest amount of exergy destruction is
Explanation of Solution
Show the T-s diagram for refrigeration system as in Figure (1).
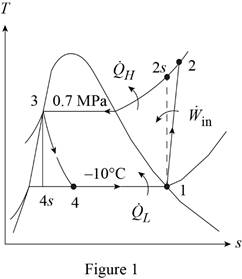
From Figure (1), write the specific enthalpy at state 3 is equal to state 4 due to throttling process.
Here, specific enthalpy at state 3 and 4 is
Express specific enthalpy at state 2 using compressor efficiency.
Here, specific enthalpy at state 2, 1 and 2s is
Express temperature at state 3.
Here, saturation temperature at pressure of
Express quality at state 4.
Here, specific enthalpy at saturation liquid and temperature of
Express specific entropy at state 4.
Here, specific entropy at saturation liquid and temperature of
Express heat rejected in the evaporator.
Here, specific enthalpy at state 4 is
Express heat added in the condenser.
Express the exergy destruction during process 1-2.
Here, surrounding temperature is
Express the exergy destruction during process 2-3.
Here, specific entropy at state 3 is
Express the exergy destruction during process 3-4.
Here, specific entropy at state 4 is
Express the exergy destruction during process 4-1.
Here, freezing temperature of water is
Conclusion:
Refer Table A-11, “saturated refrigerant-134a-temperature table”, and write the properties corresponding to initial temperature
Here, specific entropy at state 1 is
Perform unit conversion of pressure at state 2 from
Refer Table A-13, “superheated refrigerant 134a”, and write the specific enthalpy at state 2s corresponding to pressure at state 2 of
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is specific entropy at state 2 and specific enthalpy at state 2s respectively.
Show the specific enthalpy at state 2s corresponding to specific entropy as in Table (1).
|
Specific entropy at state 2 |
Specific enthalpy at state 2s |
| 0.9314 | 268.47 |
| 0.9378 | |
| 0.9642 | 278.59 |
Substitute
Thus, the specific enthalpy at state 2s is,
Refer Table A-12, “saturated refrigerant-134a-pressure table”, and write the saturated temperature corresponding to pressure of
Substitute
Refer Table A-11, “saturated refrigerant-134a-temperature table”, and write the specific enthalpy and entropy at state 3 corresponding to temperature at state 3
Here, specific enthalpy and entropy at saturated liquid is
Substitute
Substitute
Refer Table A-11, “saturated refrigerant-134a-temperature table”, and write the properties corresponding to final temperature
Substitute
Substitute
Refer Table A-13, “superheated refrigerant 134a”, and write the specific entropy at state 2 corresponding to pressure at state 2 of
Show the specific entropy at state 2 corresponding to specific enthalpy as in Table (2).
|
Specific enthalpy at state 1 |
Specific entropy at state 2 |
| 268.47 | 0.9314 |
| 275.02 | |
| 278.59 | 0.9642 |
Use excels and tabulates the values from Table (2) in Equation (XII) to get,
Substitute
Substitute
Perform unit conversion of temperature from
Substitute
Substitute
Substitute
Take the freezing temperature of water as,
Substitute
Hence, the process that causes the greatest amount of exergy destruction is
Want to see more full solutions like this?
Chapter 11 Solutions
Thermodynamics: An Engineering Approach
- Hints: Find the closed loop transfer function and then plot the step response for diFerentvalues of K in MATLAB. Show step response plot for different values of K. Auto Controls Show solutions and provide matlab code NO COPIED ANSWERS OR WILL REPORT!!!! Use own solutionarrow_forwardwhat is shear stress and normal? how to tell them while calculating?arrow_forward12 mm 45 mm 20 kN 20 kN 12 mm 45 mm PROBLEM 1.61 For the assembly and loading of Problem 1.60, determine (a) the average shearing stress in the pin at C, (b) the average bearing stress at C in member BC, (c) the average bearing stress at B in member BC. PROBLEM 1.60 Two horizontal 20-kN forces are applied to pin B of the assembly shown. Knowing that a pin of 20-mm diameter is used at each connection, determine the maximum value of the average normal stress (a) in link AB, (b) in link BC.arrow_forward
- How do you find these answers?arrow_forward250 mm 400 mm A B C E F 250 mm PROBLEM 1.52 Each of the two vertical links CF connecting the two horizontal members AD and EG has a 10 × 40-mm uniform rectangular cross section and is made of a steel with an ultimate strength in tension of 400 MPa, while each of the pins at C and F has a 20-mm diameter and are made of a steel with an ultimate strength in shear of 150 MPa. Determine the overall factor of safety for the links CF and the pins connecting them to the horizontal members. 24 kNarrow_forward50 mm 12 mm B O C OA 300 mm 450 mm E PROBLEM 1.51 Each of the steel links AB and CD is connected to a support and to member BCE by 25-mm-diameter steel pins acting in single shear. Knowing that the ultimate shearing stress is 210 MPa for the steel used in the pins and that the ultimate normal stress is 490 MPa for the steel used in the links, determine the allowable load P if an overall factor of safety of 3.0 is desired. (Note that the links are not reinforced around the pin holes.)arrow_forward
- 3. A 15% magnesium chloride solution is flowing through a 5-nom sch 40 commercial steel pipe at a rate of 325,000 lbm/h. The average temperature of the magnesium chloride solution as it flows through the pipe is 10°F. Determine the convective heat transfer coefficient inside the pipe.arrow_forward2. Jojoba oil is flowing through a ¾-nom stainless steel pipe at a flow rate of 1,850 lbm/h. After the velocity profile in the pipe is fully developed, the oil enters a heater, as shown in Figure P5.7. The length of the heater section is 5 ft. The properties of the jojoba oil at the average temperature in the heater section are given in Table P5.7. Determine the convective heat transfer coefficient inside the heater section of the pipe. ¾ nom stainless steel pipe Heater section L=5ft Fig. P5.7 TABLE P5.7 Thermophysical Properties of Jojoba Oil at the Average Temperature in the Heater P (lbm/ft³) 68.671 (Btu/lbm-R) 0.30339 μ (lbm/ft-s) 0.012095 k (Btu/h-ft-°F) 0.077424arrow_forward1. Water is flowing inside of a 3-std type K copper tube at a flow rate of 1.2 kg/s. The average temperature of the water is 50°C. Cold, dry air at a temperature of 5°C and atmospheric pressure flows outside of the tube in cross flow with a velocity of 85 m/s. Determine the UA product for this tube under clean conditions.arrow_forward
- Hints: Find the closed loop transfer function and then plot the step response for diFerentvalues of K in MATLAB. Show step response plot for different values of K. Auto Controls Show solutions and provide matlab code NO COPIED ANSWERS OR WILL REPORT!!!!arrow_forward37. The vertical shaft shown in Figure P12-37 is driven at a speed of 600 rpm with 4.0 hp entering through the bevel gear. Each of the two chain sprockets delivers 2.0 hp to the side to drive mixer blades in a chemical reactor vessel. The bevel gear has a diametral pitch of 5, a pitch diameter of 9.000 in, a face width of 1.31 in, and a pressure angle of 20°. Use SAE 4140 OQT 1000 steel for the shaft. See Chapter 10 for the methods for computing the forces on the bevel gear. Figure P12-37: P37-Bevel gear drive with two chain sprockets Each problem includes the following details: ■Design the complete shaft, including the specification of the overall geometry and the consideration of stress con- centration factors. The analysis would show the minimum acceptable diameter at each point on the shaft to be safe from the standpoint of strength. Homework Problems 12-24, 12-35, and 12-37 from textbook, done in spreadsheet form. Place drawings of the load, shear, and bending moment body diagrams…arrow_forward35. The double-reduction, helical gear reducer shown in Figure P12-35 transmits 5.0 hp. Shaft 1 is the input, rotating at 1800 rpm and receiving power directly from an electric motor through a flexible coupling. Shaft 2 rotates at 900 rpm. Shaft 3 is the output, rotating at 300 rpm. A chain sprocket is mounted on the output shaft as shown and delivers the power upward. The data for the gears are given in Table 12-5. Each gear has a 1412° normal pressure angle and a 45° helix angle. The combinations of left- and right-hand helixes are arranged so that the axial forces oppose each other on shaft 2 as shown. Use SAE 4140 OQT 1200 for the shafts. Figure P12-35: P35-Double-reduction helical drive Each problem includes the following details: ■Design the complete shaft, including the specification of the overall geometry and the consideration of stress con- centration factors. The analysis would show the minimum acceptable diameter at each point on the shaft to be safe from the standpoint of…arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





