
Thermodynamics: An Engineering Approach
9th Edition
ISBN: 9781259822674
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.11, Problem 99RP
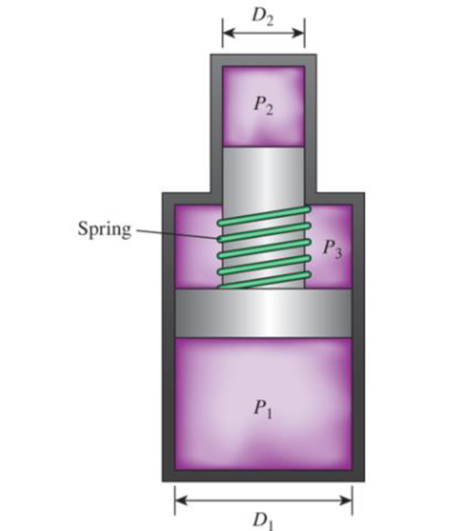
The force generated by a spring is given by F = kx, where k is the spring constant and x is the deflection of the spring. The spring of Fig. 1–99 has a spring constant of 8 kN/cm. The pressures are P1 = 5000 kPa, P2 = 10,000 kPa, and P3 = 1000 kPa. If the piston diameters are D1 = 8 cm and D2 = 3 cm, how far will the spring be deflected? Answer: 1.72 cm
 c
c
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
4. The figure below shows a bent pipe with the external loading FA
228 lb, and M₁ = M₂ = 1 kip-ft. The force Fernal loading FA = 300 lb, FB:
parallel to the y-axis, and
and yc = 60°.
= 125 lb, Fc
=
acts parallel to the x-z plane, the force FB acts
Cartesian resultan Coordinate direction angles of Fc are ac = 120°, ẞc = 45°,
a. Compute the resultant force vector of the given external loading and express it in
EST
form.
b. Compute the resultant moment vector of the given external loading about the origin, O,
and express it in Cartesian vector form. Use the vector method while computing the
moments of forces.
c. Compute the resultant moment vector of the given external loading about the line OA
and express it in Cartesian vector form.
:00 PM EST
k
ghoufran@buffaternal du
2 ft
M₁
A
40°
FA
M2
C
18 in
1 ft
Fc
25
houfran@bald.edu - Feb 19,
3 ft
FB
The differential equation of a cruise control system is provided by the following equation:
Find the closed loop transfer function with respect to the reference velocity (vr) .
a. Find the poles of the closed loop transfer function for different values of K. How does the poles move as you change K?
b. Find the step response for different values of K and plot in MATLAB. What can you observe?
c. For the given transfer function, find tp, ts, tr, Mp . Plot the resulting step response. G(s) = 40/(s^2 + 4s + 40)
Auto Controls
Perform the partial fraction expansion of the following transfer function and find the impulse response:
G(s) = (s/2 + 5/3) / (s^2 + 4s + 6)
G(s) =( 6s^2 + 50) / (s+3)(s^2 +4)
Chapter 1 Solutions
Thermodynamics: An Engineering Approach
Ch. 1.11 - The value of the gravitational acceleration g...Ch. 1.11 - One of the most amusing things a person can...Ch. 1.11 - An office worker claims that a cup of cold coffee...Ch. 1.11 - What is the difference between the classical and...Ch. 1.11 - Explain why the light-year has the dimension of...Ch. 1.11 - What is the difference between pound-mass and...Ch. 1.11 - What is the net force acting on a car cruising at...Ch. 1.11 - What is the weight, in N, of an object with a mass...Ch. 1.11 - If the mass of an object is 10 lbm, what is its...Ch. 1.11 - The acceleration of high-speed aircraft is...
Ch. 1.11 - The value of the gravitational acceleration g...Ch. 1.11 - A 3-kg plastic tank that has a volume of 0.2 m3 is...Ch. 1.11 - A 2-kg rock is thrown upward with a force of 200 N...Ch. 1.11 - Solve Prob. 113 using appropriate software. Print...Ch. 1.11 - A 4-kW resistance heater in a water heater runs...Ch. 1.11 - A 150-lbm astronaut took his bathroom scale (a...Ch. 1.11 - The gas tank of a car is filled with a nozzle that...Ch. 1.11 - How would you define a system to determine the...Ch. 1.11 - A large fraction of the thermal energy generated...Ch. 1.11 - A can of soft drink at room temperature is put...Ch. 1.11 - How would you define a system to determine the...Ch. 1.11 - How would you describe the state of the air in the...Ch. 1.11 - What is the difference between intensive and...Ch. 1.11 - The specific weight of a system is defined as the...Ch. 1.11 - Is the number of moles of a substance contained in...Ch. 1.11 - Is the state of the air in an isolated room...Ch. 1.11 - What is a quasi-equilibrium process? What is its...Ch. 1.11 - Define the isothermal, isobaric, and isochoric...Ch. 1.11 - What is specific gravity? How is it related to...Ch. 1.11 - What are the ordinary and absolute temperature...Ch. 1.11 - Consider an alcohol and a mercury thermometer that...Ch. 1.11 - Consider two dosed systems A and B. System A...Ch. 1.11 - Consider a system whose temperature is 18C....Ch. 1.11 - Steam enters a heat exchanger at 300 K. What is...Ch. 1.11 - The temperature of a system rises by 130C during a...Ch. 1.11 - The temperature of a system drops by 45F during a...Ch. 1.11 - The temperature of the lubricating oil in an...Ch. 1.11 - Heated air is at 150C. What is the temperature of...Ch. 1.11 - What is the difference between gage pressure and...Ch. 1.11 - Explain why some people experience nose bleeding...Ch. 1.11 - A health magazine reported that physicians...Ch. 1.11 - Someone claims that the absolute pressure in a...Ch. 1.11 - Consider two identical fans, one at sea level and...Ch. 1.11 - The absolute pressure in a compressed air tank is...Ch. 1.11 - A manometer measures a pressure difference as 40...Ch. 1.11 - A vacuum gage connected to a chambee reads 35 kPa...Ch. 1.11 - The maximum safe air pressure of a tire is...Ch. 1.11 - A pressure gage connected to a tank reads 50 psi...Ch. 1.11 - A pressure gage connected to a tank reads 500 kPa...Ch. 1.11 - A 200-pound man has a total foot imprint area of...Ch. 1.11 - The gage pressure in a liquid at a depth of 3 m is...Ch. 1.11 - The absolute pressure in water at a depth of 9 m...Ch. 1.11 - Consider a 1.75-m-tall man standing vertically in...Ch. 1.11 - The barometer of a mountain hiker reads 750 mbars...Ch. 1.11 - The basic barometer can be used to measure the...Ch. 1.11 - A gas is contained in a vertical, frictionless...Ch. 1.11 - Reconsider Prob. 158. Using appropriate software,...Ch. 1.11 - The piston of a vertical piston-cylinder device...Ch. 1.11 - Both a gage and a manometer are attached to a gas...Ch. 1.11 - Reconsider Prob. 161. Using appropriate software,...Ch. 1.11 - A manometer containing oil ( = 850 kg/m3) is...Ch. 1.11 - A manometer is used to measure the air pressure in...Ch. 1.11 - A mercury manometer ( = 13.600 kg/m3) is connected...Ch. 1.11 - Repeat Prob. 165 for a differential mercury height...Ch. 1.11 - The pressure in a natural gas pipeline is measured...Ch. 1.11 - Repeat Prob. 167E by replacing air with oil with a...Ch. 1.11 - Blood pressure is usually measure by wrapping a...Ch. 1.11 - The maximum blood pressure in the upper arm of a...Ch. 1.11 - Consider a U-tube whose arms are open to the...Ch. 1.11 - Consider a double-fluid manometer attached to an...Ch. 1.11 - Calculate the absolute pressure. P1, of the...Ch. 1.11 - Consider the manometer in Fig. 173. If the...Ch. 1.11 - Consider the manometer in Fig. 173. If the...Ch. 1.11 - The hydraulic lift in a car repair shop has an...Ch. 1.11 - Consider the system shown in Fig. 177. If a change...Ch. 1.11 - The gage pressure of the air in the tank shown in...Ch. 1.11 - Repeat Prob. 178 for a gage pressure of 40 kPa.Ch. 1.11 - What is the value of the engineering software...Ch. 1.11 - Determine a positive real root of this equation...Ch. 1.11 - Solve this system of two equations with two...Ch. 1.11 - Solve this system of three equations with three...Ch. 1.11 - Solve this system of three equations with three...Ch. 1.11 - The reactive force developed by a jet engine to...Ch. 1.11 - The reactive force developed by a jet engine to...Ch. 1.11 - A man goes to a traditional market to buy a steak...Ch. 1.11 - What is the weight of a 1-kg substance in N, kN,...Ch. 1.11 - The pressure in a steam boiler is given to be 92...Ch. 1.11 - A hydraulic lift is to be used to lift a 1900-kg...Ch. 1.11 - The average atmosphere pressure on earth is...Ch. 1.11 - Hyperthermia of 5C (i.e., 5C rise above the normal...Ch. 1.11 - The boiling temperature of water decreases by...Ch. 1.11 - A house is losing heat at a rate of 1800 kJ/h per...Ch. 1.11 - The average body temperature of a person rises by...Ch. 1.11 - The average temperature of the atmosphere in the...Ch. 1.11 - A vertical, frictionless pistoncylinder device...Ch. 1.11 - A vertical pistoncylinder device contains a gas at...Ch. 1.11 - The force generated by a spring is given by F =...Ch. 1.11 - An air-conditioning system requires a 35-m-long...Ch. 1.11 - Balloons are often filled with helium gas because...Ch. 1.11 - Reconsider Prob. 1101. Using appropriate software,...Ch. 1.11 - Determine the maximum amount of load, in kg, the...Ch. 1.11 - The lower half of a 6-m-high cylindrical container...Ch. 1.11 - A pressure cooker cooks a lot faster than an...Ch. 1.11 - The pilot of an airplane reads the altitude 6400 m...Ch. 1.11 - A glass tube is attached to a water pipe, as shown...Ch. 1.11 - Consider a U-tube whose arms are open to the...Ch. 1.11 - A water pipe is connected to a double-U manometer...Ch. 1.11 - A gasoline line is connected to a pressure gage...Ch. 1.11 - Repeat Prob. 1110 for a pressure gage reading of...Ch. 1.11 - When measuring small pressure differences with a...Ch. 1.11 - Pressure transducers are commonly used to measure...Ch. 1.11 - Consider the flow of air through a wind turbine...Ch. 1.11 - The drag force exerted on a car by air depends on...Ch. 1.11 - It is well known that cold air feels much colder...Ch. 1.11 - Reconsider Prob. 1116E. Using appropriate...Ch. 1.11 - During a heating process, the temperature of an...Ch. 1.11 - An apple loses 3.6 kJ of heat as it cools per C...Ch. 1.11 - At sea level, the weight of 1 kg mass in SI units...Ch. 1.11 - Consider a fish swimming 5 m below the free...Ch. 1.11 - The atmospheric pressures at the top and the...Ch. 1.11 - Consider a 2.5-m-deep swimming pool. The pressure...
Additional Engineering Textbook Solutions
Find more solutions based on key concepts
1.2 Explain the difference between geodetic and plane
surveys,
Elementary Surveying: An Introduction To Geomatics (15th Edition)
How are relationships between tables expressed in a relational database?
Modern Database Management
How is the hydrodynamic entry length defined for flow in a pipe? Is the entry length longer in laminar or turbu...
Fluid Mechanics: Fundamentals and Applications
The solid steel shaft AC has a diameter of 25 mm and is supported by smooth bearings at D and E. It is coupled ...
Mechanics of Materials (10th Edition)
How does a computers main memory differ from its auxiliary memory?
Java: An Introduction to Problem Solving and Programming (8th Edition)
17–1C A high-speed aircraft is cruising in still air. How does the temperature of air at the nose of the aircra...
Thermodynamics: An Engineering Approach
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Study Area Document Sharing User Settings mylabmastering.pearson.com Access Pearson P Pearson MyLab and Mastering The 150-lb skater passes point A with a speed of 6 ft/s. (Figure 1) Figure 1 of 1 Part A P Course Home b My Questions | bartleby Determine his speed when he reaches point B. Neglect friction. Express your answer to three significant figures and include the appropriate units. με ? VB = Value Units Submit Request Answer Part B Determine the normal force exerted on him by the track at this point. Express your answer to three significant figures and include the appropriate units. ☐ о Α NB = Value Units Submit Request Answer Provide Feedback ? ■Review Next >arrow_forwardmylabmastering.pearson.com Access Pearson P Pearson MyLab and Mastering P Course Home b My Questions | bartleby Study Area Document Sharing User Settings The 100-kg crate is subjected to the forces shown. The crate is originally at rest. The coefficient of kinetic friction between the crate and the surface is μk = 0.2. (Figure 1) Part A Determine the distance it slides in order to attain a speed of 8.1 m/s. Express your answer to three significant figures and include the appropriate units. Figure 500 N 1 of 1 Α S = Value Units Submit Request Answer Provide Feedback ? ■Review Next >arrow_forwardThe differential equation of a DC motor can be described by the following equation Find the transfer function between the applied voltage ( Va)and the motor speed (thetadot m). What is the steady state speed of the motor after a voltage (Va = 10V) has been applied. Find the transfer function between the applied voltage (Va) and the shaft angle (thetadot m) .arrow_forward
- Study Area Document Sharing User Settings Access Pearson mylabmastering.pearson.com P Pearson MyLab and Mastering The crash cushion for a highway barrier consists of a nest of barrels filled with an impact-absorbing material. The barrier stopping force is measured versus the vehicle penetration into the barrier. (Figure 1) Part A P Course Home b My Questions | bartleby Review Determine the distance a car having a weight of 4000 lb will penetrate the barrier if it is originally traveling at 55 ft/s when it strikes the first barrel. Express your answer to three significant figures and include the appropriate units. Figure 1 of 1 36 μΑ S = Value Units Submit Request Answer Provide Feedback ? Next >arrow_forwardStudy Area Document Sharing User Settings mylabmastering.pearson.com Access Pearson P Pearson MyLab and Mastering Part A P Course Home b My Questions | bartleby ■Review The sports car has a mass of 2.5 Mg and accelerates at 6 m/s², starting from rest. (Figure 1) If the drag resistance on the car due to the wind is FD = (10v) N, where v is the velocity in m/s, determine the power supplied to the engine when t = 5 s. The engine has a running efficiency of € = 0.66. Express your answer to three significant figures and include the appropriate units. Figure 1 of 1 о Α ? P = Value Units Submit Request Answer Return to Assignment Provide Feedbackarrow_forwardAccess Pearson Study Area mylabmastering.pearson.com P Pearson MyLab and Mastering Document Sharing User Settings The car in (Figure 1) having a mass of 2 Mg is originally traveling at 2 m/s. Assume 0 = 22°. Figure 1 of 1 Part A P Course Home b My Questions | bartleby ■Review Determine the distance it must be towed by a force F = 4 kN in order to attain a speed of 6 m/s. Neglect friction and the mass of the wheels. Express your answer to three significant figures and include the appropriate units. Α ? S = Value Units Submit Request Answer Provide Feedback Next >arrow_forward
- Derive the Laplace transform of the following functions. Use the definition of Laplace transform. f(t)=sin4t and f(t)=cos2t Auto Controlsarrow_forwardStudy Area Document Sharing User Settings Access Pearson P Pearson MyLab and Mastering Marbles having a mass of 5 g fall from rest at A through the glass tube and accumulate in the can at C. (Figure 1) Figure Aarrow_forwardVC Vc B S TDC -BDC S TQ Tp = Pg A (asne) [1+ % CUSA] At what position (in degrees after top dead center) would you want the peak pressure of combustion to occur to create the maximum torque on the crankshaft? For a 100mm piston digimeter acting on a connecting. rod with a length of 80mm use the equation above to calculate the torque (NIM) on the crankshaft at this crank position for an engine that develops a peak pressure of 135 bararrow_forward
- Access Pearson P Pearson MyLab and Mastering Study Area Document Sharing User Settings The man having a weight of 180 lb is able to run up a 18-ft-high flight of stairs shiwn in (Figure 1) in 4 s. Figure 1 of 1 R mylabmastering.pearson.com Part A P Course Home b My Questions | bartleby Determine the power generated. Express your answer in horsepower to three significant figures. ΜΕ ΑΣΦ. Η vec P = Submit Request Answer Part B ? hp How long would a 100-W light bulb have to burn to expend the same amount of energy? Express your answer to three significant figures and include the appropriate units. HÅ ? t = Value Units Submit Request Answer Provide Feedback Review Next >arrow_forwardThe tension in the belt is 46 lb. Determine the moment of the force F1 about the pin at A. Determine the moment of the force F2 about the pin at A.arrow_forward1. Describe each of the tolerances in the following drawing: 0.01 A 09±0.025 .10±0.01 0.015 AB 6.76 08.51 03±0.05 0.015 MAB 14±0.03 60 14±0.02 12±0.08 0.01 A Barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Thermodynamics - Chapter 3 - Pure substances; Author: Engineering Deciphered;https://www.youtube.com/watch?v=bTMQtj13yu8;License: Standard YouTube License, CC-BY