
Concept explainers
(a)
Interpretation:
The formula of
Concept Introduction:
Alcohols:
Organic compounds that contain a hydroxyl group that is covalently bonded to a carbon atom
- 1) Primary alcohol: One organic group attached to hydroxyl atom.
- 2) Secondary alcohol: Two organic groups bonded to hydroxyl atom.
- 3) Tertiary alcohol: Three organic groups bonded to hydroxyl atom.
The general formula for alcohol is,
Primary alcohol:
Secondary alcohol:
Tertiary alcohol:
Phenol:
Organic compounds those in which, a hydroxyl group is attached directly to an
(a)
Answer to Problem 11D.7E
The formula of
Explanation of Solution
The structure of
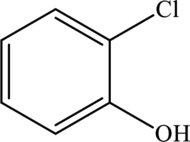
One-chlorine substituent is attached to the benzene ring in the first position and the hydroxyl group is attached to the benzene ring in second position. The formula of
(b)
Interpretation:
The formula of
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 11D.7E
The formula of
Explanation of Solution
The structure of
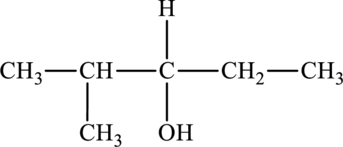
The parent chain is pentane. A hydroxyl group is present at the carbon third position and one methyl group is present in the carbon second position. The formula of
(c)
Interpretation:
The formula of
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 11D.7E
The formula of
Explanation of Solution
The structure of
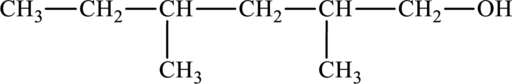
The parent chain is hexane. A hydroxyl group is present at the carbon first position and two methyl groups are present in the carbon second position and fourth position. The formula of
(d)
Interpretation:
The formula of
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 11D.7E
The formula of
Explanation of Solution
The structure of
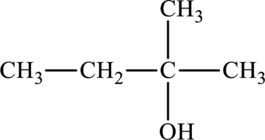
The parent chain is butane. A hydroxyl group is present at the carbon second position and one methyl group is present in the carbon second position. The formula of
Want to see more full solutions like this?
Chapter 11 Solutions
Chemical Principles: The Quest for Insight
- H-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 H-CI CH2Cl2 CIarrow_forwardDraw the products of the stronger acid protonating the other reactant. དའི་སྐད”“ H3C OH H3C CH CH3 KEq Product acid Product basearrow_forward
- Draw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
