
Consider a steam power plant that operates on a reheat Rankine cycle. Steam enters the high-pressure turbine at 10 MPa and 500°C and the low-pressure turbine at 1 MPa and 500°C. Steam leaves the condenser as a saturated liquid at a pressure of 10 kPa. The isentropic efficiency of the turbine is 80 percent, and that of the pump is 95 percent. Determine the exergy destruction associated with the heat addition process and the expansion process. Assume a source temperature of 1600 K and a sink temperature of 285 K. Also, determine the exergy of the steam at the boiler exit. Take P0 = 100 kPa.
The exergy destruction associated with the heat addition, the expansion process, and the exergy of the steam at the boiler exit.
Answer to Problem 62P
The exergy destruction during the heating process is
The exergy destruction during the expansion process is
The exergy of the steam at the boiler exit is
Explanation of Solution
Draw the
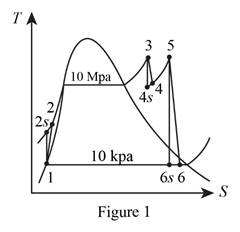
Here, water is the working fluid.
Write the formula for work done by the pump during process 1-2 with the consideration of isentropic efficiency
Here, the specific volume is
Write the formula for enthalpy
Before reheating,
At state
The isentropic efficiency is expressed as follows.
After reheating,
At state 5:
The reheating occurs at constant pressure. Hence, the pressure at state 4 and state 5 are equal.
At state
The steam is expanded to the pressure of
The quality of water at the exit of the L.P turbine (state 6) is expressed as follows (actual).
The enthalpy at state 6 is expressed as follows.
Here, the enthalpy is
The isentropic efficiency is expressed as follows.
Here, the subscript
Write the formula for heat input
Write the formula for the exergy destruction for the combined heat addition and pumping process 1-5.
Here, the entropy
Write the formula for the exergy destruction for pumping process 1-2.
Here, the work input of pump at actual process 1-2 is
Write the formula for exergy destruction for heat addition processes 2-3, 4-5.
Write the formula for the exergy destruction for the expansion process 3-4, 5-6.
Write the formula for exergy of the steam at boiler exit
Here, the enthalpy is
Neglect the kinetic energy
At state 1:
The water exits the condenser as a saturated liquid at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
At state 3: (H.P. Turbine inlet)
The steam enters the as superheated vapor.
Refer Table A-6, “Superheated water”.
The enthalpy
At state
From Figure 1.
Refer Table A-6, “Superheated water”.
The enthalpy
At state 5: (L.P. Turbine inlet)
The steam is reheated to superheated at the pressure of
Refer Table A-6, “Superheated water”.
The enthalpy
At state
From Figure 1.
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following properties corresponding to the pressure of
Here, the sink temperature is equal to the surrounding temperature.
The surrounding pressure
Refer Table A-4, “Saturated water-Temperature table”.
The enthalpy
Conclusion:
Substitute
Substitute
Substitute
Substitute
Substitute
Equation (V).
Substitute
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the vapor enthalpy
The calculated enthalpy at state 6 is greater than the vapor enthalpy at this state. Hence, the steam is at superheated state.
Substitute
Consider the process 1 to 5 (combined heat addition and boiler).
Here,
Substitute 285 K for
Substitute
Substitute
Thus, the exergy destruction during the heating process is
Consider the process 3-4 and 5-6 (H.P. turbine expansion and L.P. turbine expansion).
Here,
Substitute 285 K for
Thus, the exergy destruction during the expansion process is
Substitute
Thus, the exergy of the steam at the boiler exit is
Want to see more full solutions like this?
Chapter 10 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
- A spring package with two springs and an external force, 200N. The short spring has a loin of 35 mm. Constantly looking for spring for short spring so that total compression is 35 mm (d). Known values: Long spring: Short spring:C=3.98 N/mm Lo=65mmLo=87.4mmF=c·fTotal compression is same for both spring. 200 = (3.98(c1) × 35) + (c₂ × 35) 200 = 139.3 + 35c₂ 200 - 139.3 = 35c₂ 60.7 = 35c₂ c₂ = 60.7/35 Short spring (c₂) = 1.73 N/mm According to my study book, the correct answer is 4.82N/mm What is wrong with the calculating?arrow_forwardWhat is the reason for this composition?arrow_forwardHomework: ANOVA Table for followed design B AB Dr -1 -1 1 (15.18,12) 1 -1 -1 (45.48.51) -1 1 -1 (25,28,19) 1 1 (75.75,81)arrow_forward
- 20. [Ans. 9; 71.8 mm] A semi-elliptical laminated spring is made of 50 mm wide and 3 mm thick plates. The length between the supports is 650 mm and the width of the band is 60 mm. The spring has two full length leaves and five graduated leaves. If the spring carries a central load of 1600 N, find: 1. Maximum stress in full length and graduated leaves for an initial condition of no stress in the leaves. 2. The maximum stress if the initial stress is provided to cause equal stress when loaded. [Ans. 590 MPa ; 390 MPa ; 450 MPa ; 54 mm] 3. The deflection in parts (1) and (2).arrow_forwardQ6/ A helical square section spring is set inside another, the outer spring having a free length of 35 mm greater than the inner spring. The dimensions of each spring are as follows: Mean diameter (mm) Side of square section (mm) Active turns Outer Inner Spring Spring 120 70 8 7 20 15 Determine the (1) Maximum deflection of the two springs and (2) Equivalent spring rate of the two springs after sufficient load has been applied to deflect the outer spring 60 mm. Use G = 83 GN/m².arrow_forwardQ2/ The bumper springs of a railway carriage are to be made of rectangular section wire. The ratio of the longer side of the wire to its shorter side is 1.5, and the ratio of mean diameter of spring to the longer side of wire is nearly equal to 6. Three such springs are required to bring to rest a carriage weighing 25 kN moving with a velocity of 75 m/min with a maximum deflection of 200 mm. Determine the sides of the rectangular section of the wire and the mean diameter of coils when the shorter side is parallel to the axis of the spring. The allowable shear stress is not to exceed 300 MPa and G = 84 kN/mm². Q6/ A belicalarrow_forward
- 11. A load of 2 kN is dropped axially on a close coiled helical spring, from a height of 250 mm. The spring has 20 effective turns, and it is made of 25 mm diameter wire. The spring index is 8. Find the maximum shear stress induced in the spring and the amount of compression produced. The modulus of rigidity for the material of the spring wire is 84 kN/mm². [Ans. 287 MPa; 290 mm]arrow_forwardWhat is the reason for this composition?arrow_forwardHomework: ANOVA Table for followed design B AB Dr -1 -1 1 (15.18,12) 1 -1 -1 (45.48.51) -1 1 -1 (25,28,19) 1 1 (75.75,81)arrow_forward
- S B Pin 6 mm Garrow_forwardMid-Term Exam 2024/2025 Post graduate/Applied Mechanics- Metallurgy Q1/ State the type of fault in the following case, and state the structure in which it will appear. АВСАВСВАСВАСАВСАВСarrow_forwardالثانية Babakt Momentum equation for Boundary Layer S SS -Txfriction dray Momentum equation for Boundary Layer What laws are important for resolving issues 2 How to draw. 3 What's Point about this.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





