
Concept explainers
(a)
Interpretation:
Among the following compounds the compound which reacts faster in
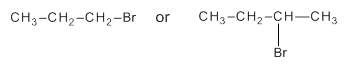
Concept Introduction:
Substitution reaction is a reaction in which an atom or group of a molecule is replaced by another atom or group.
SN2 reaction refers to bimolecular substitution
(b)
Interpretation:
Among the following compounds the compound which reacts faster in
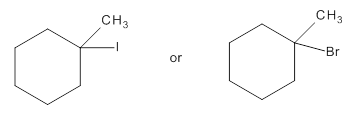
Concept Introduction:
For an E1/E2 reaction, when same alkyl group is present with the leaving group, the relative reactivity of
(c)
Interpretation:
Among the following which reacts faster in SN1 reaction is to be identified.
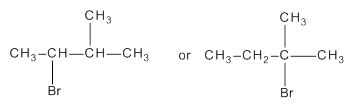
Concept Introduction:
Substitution reaction is a reaction in which an atom or group of a molecule is replaced by another atom or group.
SN1 reaction refers to unimolecular substitution reaction in which rate of the reaction depends on concentration of only one reactive species.
(d)
Interpretation:
Among the following which reacts faster in E2 reaction is to be identified.
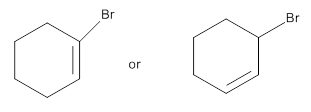
Concept Introduction:
- Elimination reaction is a reaction in which an atom or group of a molecule is eliminated and a double bond is formed.
- This type of reaction primarily occur in alkyl/aryl halides.
- Elimination reactions are of two types – E1 elimination and E2 elimination.
- E2 elimination is a concerted reaction and involves formation of transition state in which both proton and halide are removed in one single step.
- E2 elimination follows Zaistev’s rule that the major product is usually the most substituted
alkene . Thus E2 reaction is regioselective if the leaving group is too weak then least substituted alkene is formed. - Primary and secondary halides undergo E2 elimination whereas tertiary halides undergo E1 elimination as tertiary carbocation formed is more stable.
- Allylic and benzylic halides undergo both E1 and E2 reactions.
- Presence of high concentration of strong base favors E2 reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Organic Chemistry
- Answer the question in the first photoarrow_forwardGgggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forwardExplain the concepts of hemiacetal and acetal.arrow_forward
- Photochemical smog is formed in part by the action of light on nitrogen dioxide. The wavelength of radiation absorbed by NO2 in this reaction is 197 nm.(a) Draw the Lewis structure of NO2 and sketch its π molecular orbitals.(b) When 1.56 mJ of energy is absorbed by 3.0 L of air at 20 °C and 0.91 atm, all the NO2 molecules in this sample dissociate by the reaction shown. Assume that each absorbed photon leads to the dissociation (into NO and O) of one NO2 molecule. What is the proportion, in parts per million, of NO2 molecules in this sample? Assume that the sample behaves ideally.arrow_forwardCorrect each molecule in the drawing area below so that it has the skeletal ("line") structure it would have if it were dissolved in a 0.1 M aqueous solution of HCI. If there are no changes to be made, check the No changes box under the drawing area. No changes. HO Explanation Check NH, 2 W O :□ G ©2025 M unter Accessibilityarrow_forwardAn expression for the root mean square velocity, vrms, of a gas was derived. Using Maxwell’s velocity distribution, one can also calculate the mean velocity and the most probable velocity (mp) of a collection of molecules. The equations used for these two quantities are vmean=(8RT/πM)1/2 and vmp=(2RT/M)1/2 These values have a fixed relationship to each other.(a) Arrange these three quantities in order of increasing magnitude.(b) Show that the relative magnitudes are independent of the molar mass of the gas.(c) Use the smallest velocity as a reference for establishing the order of magnitude and determine the relationship between the larger and smaller values.arrow_forward
- The reaction of solid dimethylhydrazine, (CH3)2N2H2, and liquefied dinitrogen tetroxide, N2O4, has been investigated for use as rocket fuel. The reaction produces the gases carbon dioxide (CO2), nitrogen (N2), and water vapor (H2O), which are ejected in the exhaust gases. In a controlled experiment, solid dimethylhydrazine was reacted with excess dinitrogen tetroxide, and the gases were collected in a closed balloon until a pressure of 2.50 atm and a temperature of 400.0 K were reached.(a) What are the partial pressures of CO2, N2, and H2O?(b) When the CO2 is removed by chemical reaction, what are the partial pressures of the remaining gases?arrow_forwardOne liter of chlorine gas at 1 atm and 298 K reacts completely with 1.00 L of nitrogen gas and 2.00 L of oxygen gas at the same temperature and pressure. A single gaseous product is formed, which fills a 2.00 L flask at 1.00 atm and 298 K. Use this information to determine the following characteristics of the product:(a) its empirical formula;(b) its molecular formula;(c) the most favorable Lewis formula based on formal charge arguments (the central atom is N);(d) the shape of the molecule.arrow_forwardHow does the square root mean square velocity of gas molecules vary with temperature? Illustrate this relationship by plotting the square root mean square velocity of N2 molecules as a function of temperature from T=100 K to T=300 K.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





