
Concept explainers
Draw the major product obtained when each of the following
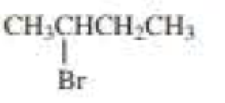
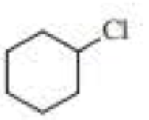
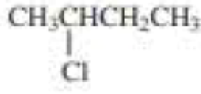
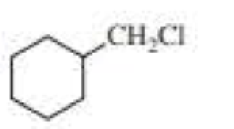
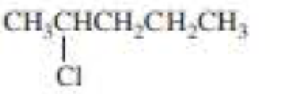
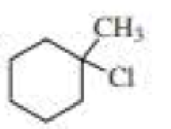
(a)
Interpretation:
Major product formed when given alkyl halides undergoes E2 reaction has to be drawn.
Concept Introduction:
An E2 reaction is a concerted, one-step reaction in which the proton and the halide ion are removed in the same step. A strong base favours an E2 reaction. A high concentration of a base favours an E2 reaction. If one of the reactants is charged, an E2 reaction will be favoured by the least polar solvent that will dissolve the reactant. If neither of the reactant is charged an E2 reaction is favoured by the protic polar solvent. The major product is a stable alkene, unless the reactants are sterically hindered.
Explanation of Solution
The product when given compound undergo E2 addition,
For an E2 reaction major product is the more stable alkene. The reactant is a secondary alkyl halide, undergo E2 reactions.
(b)
Interpretation:
Major product formed when given alkyl halides undergoes E2 reaction is to be identified.
Concept Introduction:
An E2 reaction is a concerted, one-step reaction in which the proton and the halide ion are removed in the same step.
Explanation of Solution
Chlorocyclohexane is a less stable conformer undergo E2 reaction as
The reactant is a secondary alkyl halide, undergo E2 reactions.
(c)
Interpretation:
Major product formed when given alkyl halides undergoes E2 reaction is to be identified.
Concept Introduction:
An E2 reaction is a concerted, one-step reaction in which the proton and the halide ion are removed in the same step. A strong base favours an E2 reaction. A high concentration of a base favours an E2 reaction. If one of the reactants is charged, an E2 reaction will be favoured by the least polar solvent that will dissolve the reactant. If neither of the reactant is charged an E2 reaction is favoured by the protic polar solvent. The major product is a stable alkene, unless the reactants are sterically hindered.
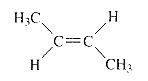
Explanation of Solution
Product of given compound when undergo E2 reaction,
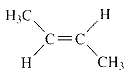
More substituted alkene is more stable therefore should be the major product.
(d)
Interpretation:
Major product formed when given alkyl halides undergoes E2 reaction is to be identified.
Concept Introduction:
An E2 reaction is a concerted, one-step reaction in which the proton and the halide ion are removed in the same step. A strong base favours an E2 reaction. A high concentration of a base favours an E2 reaction. If one of the reactants is charged, an E2 reaction will be favoured by the least polar solvent that will dissolve the reactant. If neither of the reactant is charged an E2 reaction is favoured by the protic polar solvent.
The major product is a stable alkene, unless the reactants are sterically hindered.
Explanation of Solution
Product of given compound when undergo E2 reaction,
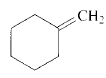
Chloromethyl cyclohexane on E2 reaction gives methylene cyclohexane. And E2 reaction is regioselective: major product is the more substituted stable alkene.
(e)
Interpretation:
Major product formed when given alkyl halides undergoes E2 reaction is to be identified.
Concept Introduction:
An E2 reaction is a concerted, one-step reaction in which the proton and the halide ion are removed in the same step. A strong base favours an E2 reaction. A high concentration of a base favours an E2 reaction. If one of the reactants is charged, an E2 reaction will be favoured by the least polar solvent that will dissolve the reactant. If neither of the reactant is charged an E2 reaction is favoured by the protic polar solvent.
The major product is a stable alkene, unless the reactants are sterically hindered.
Explanation of Solution
Product of given compound when undergo E2 reaction,
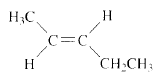
The reactant is a secondary alkyl halide, undergo E2 reactions.
(f)
Interpretation:
Major product formed when given alkyl halides undergoes E2 reaction is to be identified.
Concept Introduction:
An E2 reaction is a concerted, one-step reaction in which the proton and the halide ion are removed in the same step. A strong base favours an E2 reaction. A high concentration of a base favours an E2 reaction. If one of the reactants is charged, an E2 reaction will be favoured by the least polar solvent that will dissolve the reactant. If neither of the reactant is charged an E2 reaction is favoured by the protic polar solvent.
The major product is a stable alkene, unless the reactants are sterically hindered.
Explanation of Solution
Product of given compound when undergo E2 reaction,
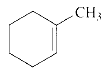
An E2 reaction is regioselective: major product is the more substituted stable alkene.
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry
- true or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forward
- true or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forward
- true or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forwardthe decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward
- 20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forwardin the following reaction, the OH- acts as which of these?NO2- (aq) + H2O (l) ⇌ OH- (aq) + HNO2 (aq)a) not a weak acidb) basec) acidarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
