
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.10, Problem 32P
What product is obtained when the following compound undergoes two successive elimination reactions?
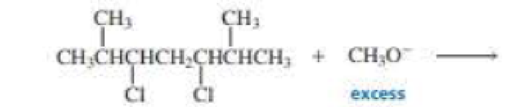
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
Chapter 10 Solutions
Organic Chemistry
Ch. 10.2 - Prob. 1PCh. 10.2 - Prob. 2PCh. 10.2 - Prob. 3PCh. 10.2 - Prob. 4PCh. 10.3 - Four alkenes are formed from the E1 reaction of...Ch. 10.3 - If 2-fluoropentane could undergo an E1 reaction,...Ch. 10.3 - Prob. 7PCh. 10.3 - Propose a mechanism for the following reaction:Ch. 10.4 - Prob. 9PCh. 10.4 - What products will be obtained from the El...
Ch. 10.4 - Prob. 11PCh. 10.5 - Prob. 12PCh. 10.6 - Prob. 14PCh. 10.7 - Why do cis-1-bromo-2-ethylcyclohexane and...Ch. 10.7 - Which isomer reacts more rapidly in an E2...Ch. 10.7 - Prob. 18PCh. 10.8 - Prob. 19PCh. 10.8 - Prob. 20PCh. 10.9 - Prob. 21PCh. 10.9 - Explain why only a substitution product and no...Ch. 10.9 - Prob. 23PCh. 10.9 - Prob. 24PCh. 10.9 - Prob. 25PCh. 10.9 - a. Explain why 1-bromo-2,2-dimethylpropane has...Ch. 10.10 - A small amount of another organic product is...Ch. 10.10 - What is the best way to prepare the following...Ch. 10.10 - Prob. 29PCh. 10.10 - Prob. 30PCh. 10.10 - Why is a cumulated diene not formed in the...Ch. 10.10 - What product is obtained when the following...Ch. 10.11 - Prob. 33PCh. 10.11 - Prob. 34PCh. 10 - Draw the major product obtained when each of the...Ch. 10 - Prob. 36PCh. 10 - a. Indicate how each of the following factors...Ch. 10 - Prob. 38PCh. 10 - A chemist wanted to synthesize the...Ch. 10 - Prob. 40PCh. 10 - Prob. 41PCh. 10 - Prob. 42PCh. 10 - Starting with an alkyl halide, how could the...Ch. 10 - Indicate which species in each pair gives a higher...Ch. 10 - Prob. 45PCh. 10 - For each of the following alkyl halides, indicate...Ch. 10 - Prob. 47PCh. 10 - When 2-bromo-2,3-dimethylbutane reacts with a...Ch. 10 - Prob. 49PCh. 10 - When the following compound undergoes solvolysis...Ch. 10 - cis-1-Bromo-4-tert-butylcyclohexane and...Ch. 10 - Draw the substitution and elimination products.Ch. 10 - Prob. 53PCh. 10 - Prob. 54PCh. 10 - Which of the following hexachlorocyclohexanes is...Ch. 10 - Explain why the rate of the reaction of...Ch. 10 - Prob. 57PCh. 10 - Two elimination products are obtained from the...Ch. 10 - Draw the structures or the product of the obtained...Ch. 10 - How could you prepare the following compounds from...Ch. 10 - cis-4-Bromocyclohexanol and...Ch. 10 - Prob. 62PCh. 10 - Prob. 63P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY