
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
8th Edition
ISBN: 9780134595450
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 10.10, Problem 43P
Interpretation Introduction
Interpretation:
The reason should be explained for the protonated tertiary
Concept introduction:
Hofmann elimination:
Quartnary ammonium ion undergoes an elimination, when using strong base like hydroxide ion this reaction is called as hofmann elimination.
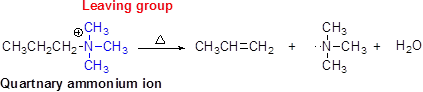
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet.
Nernst Equation: E = Eo + m (ln a)
Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ug
a)
b)
c)
H
NaOH
heat, dehydration
+
KOH
heat, dehydration
NaOH
+
(CH3)3CCHO
heat, dehydration
Ph
show mechanism
Chapter 10 Solutions
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
Ch. 10.1 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 10.1 - Explain the difference in reactivity between...Ch. 10.1 - Prob. 5PCh. 10.1 - Prob. 6PCh. 10.1 - Prob. 8PCh. 10.2 - Prob. 9PCh. 10.3 - Prob. 11PCh. 10.3 - Show how 1-propanol can be converted into the...Ch. 10.4 - Which of the following alcohols dehydrates the...Ch. 10.4 - Prob. 14P
Ch. 10.4 - Prob. 15PCh. 10.4 - Propose a mechanism for each of the following...Ch. 10.4 - Draw the product of each of the following...Ch. 10.4 - Explain why the following alcohols, when heated...Ch. 10.4 - What stereoisomers are formed in the following...Ch. 10.4 - Prob. 20PCh. 10.4 - What alcohol would you treat with phosphorus...Ch. 10.5 - Prob. 22PCh. 10.6 - What are the major products obtained when each of...Ch. 10.6 - Prob. 26PCh. 10.7 - Prob. 27PCh. 10.7 - Would you expect the reactivity of a five-membered...Ch. 10.7 - Prob. 29PCh. 10.7 - What products are obtained from the reaction of...Ch. 10.7 - Prob. 31PCh. 10.7 - Prob. 32PCh. 10.7 - Prob. 33PCh. 10.8 - Draw the mechanism for formation of the two...Ch. 10.8 - Prob. 35PCh. 10.8 - Prob. 36PCh. 10.8 - How do the major products obtained from...Ch. 10.8 - Explain why the two arene oxides in Problem 38...Ch. 10.8 - Which compound is more likely to be carcinogenic?Ch. 10.8 - Three arene oxides can be obtained from...Ch. 10.9 - Explain why the half-life (the time it takes for...Ch. 10.10 - Prob. 43PCh. 10.10 - Prob. 44PCh. 10.10 - Prob. 45PCh. 10.10 - Prob. 46PCh. 10.10 - Prob. 47PCh. 10.10 - Describe a synthesis for each of the following...Ch. 10.11 - Using an alkyl halide and a thiol as starting...Ch. 10.11 - The following three nitrogen mustards were studied...Ch. 10.11 - Why is melphalan a good cancer drug?Ch. 10.11 - Prob. 53PCh. 10.12 - Propose a mechanism for the following reaction:Ch. 10 - Prob. 55PCh. 10 - Which compound is more likely to be carcinogenic?Ch. 10 - Prob. 57PCh. 10 - Prob. 58PCh. 10 - When heated with H2SO4, both...Ch. 10 - What is the major product obtained from the...Ch. 10 - Write the appropriate reagent over each arrow.Ch. 10 - What alkenes would you expect to be obtained from...Ch. 10 - Prob. 63PCh. 10 - Prob. 64PCh. 10 - When deuterated phenanthrene oxide undergoes a...Ch. 10 - An unknown alcohol with a molecular formula of...Ch. 10 - Explain why the acid-catalyzed dehydration of an...Ch. 10 - Prob. 68PCh. 10 - Prob. 69PCh. 10 - Propose a mechanism for the following reaction:Ch. 10 - What product would be formed if the four-membered...Ch. 10 - Which of the following ethers would be obtained in...Ch. 10 - Using the given starting material any necessary...Ch. 10 - Prob. 74PCh. 10 - When 3-methyl-2-butanol is heated with...Ch. 10 - Draw structures for compounds AF.Ch. 10 - Propose a mechanism for each of the following...Ch. 10 - How could you synthesize isopropyl propyl ether,...Ch. 10 - When ethyl ether is heated with excess HI for...Ch. 10 - When the following seven-membered ring alcohol is...Ch. 10 - Ethylene oxide reacts readily with HO because of...Ch. 10 - Describe how each of the following compounds could...Ch. 10 - Propose a mechanism for each of the following...Ch. 10 - Triethylene glycol is one of the products obtained...Ch. 10 - Prob. 85PCh. 10 - Propose a mechanism for the following reaction:Ch. 10 - Prob. 87PCh. 10 - An ion with a positively charged nitrogen atom in...Ch. 10 - The following reaction takes place several times...Ch. 10 - Prob. 90PCh. 10 - Propose a mechanism for each of the following...Ch. 10 - A vicinal diol has OH groups on adjacent carbons....Ch. 10 - Prob. 93PCh. 10 - Prob. 94PCh. 10 - Two stereoisomers are obtained from the reaction...Ch. 10 - Propose a mechanism for each or the following...Ch. 10 - Triethylenemelamine (TEM) is an antitumor agent....
Knowledge Booster
Similar questions
- Please draw by handarrow_forward3. Predict the major product and give a mechanism for the following reactions: (CH3)3COH/H₂SO4 a) b) NC CH₂O c) LOCH, (CH3)3COH/H2SO4 H,SO -OHarrow_forwardIndicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forward
- Predict the major products of the following organic reaction: + A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forwardPolar solutes are most likely to dissolve into _____, and _____ are most likely to dissolve into nonpolar solvents. A. nonpolar solutes; polar solvents B. nonpolar solvents; polar solvents C. polar solvents; nonpolar solutes D. polar solutes; nonpolar solventsarrow_forwardDeducing the Peactants Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Xarrow_forward
- Draw all 8 stereoisomers, circling each pair of enantiomer(s)/ mirror image compound(s)arrow_forwardBookmarks Profiles Tab Window Help Chemical Formula - Aktiv Che X + → C 11 a app.aktiv.com Google Chrome isn't your default browser Set as default Question 12 of 16 Q Fri Feb 2 Verify it's you New Chrome availabl- Write the balanced molecular chemical equation for the reaction in aqueous solution for mercury(I) nitrate and chromium(VI) sulfate. If no reaction occurs, simply write only NR. Be sure to include the proper phases for all species within the reaction. 3 Hg(NO3)2(aq) + Cг2(SO4)3(aq) → 3 Hg₂SO (s) + 2 Cr(NO3), (aq) ean Ui mate co ence an climate bility inc ulnerabili women, main critic CLIMATE-INI ernational + 10 O 2 W FEB 1 + 4- 3- 2- 2 2 ( 3 4 NS 28 2 ty 56 + 2+ 3+ 4+ 7 8 9 0 5 (s) (1) Ch O 8 9 (g) (aq) Hg NR CI Cr x H₂O A 80 Q A DII A F2 F3 FA F5 F6 F7 F8 F9 #3 EA $ do 50 % 6 CO & 7 E R T Y U 8 ( 9 0 F10 34 F11 川 F12 Subr + delete 0 { P }arrow_forwardDeducing the reactants of a Diels-Alder reaction n the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. >arrow_forward
- Predict the major products of the following organic reaction: + Some important notes: A ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure.arrow_forwardif the answer is no reaction than state that and please hand draw!arrow_forward"I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,