
(a)
Interpretation:
The mechanism of the given reaction should be proposed.
Concept introduction:
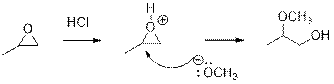
In the presence of acid catalyst, this reaction takes place through partial
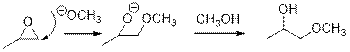
Epoxides are reactive, methoxide ion attacks epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product.
(b)
Interpretation:
The structure of all products that formed in the given reaction should be drawn.
Concept introduction:

In the presence of acid catalyst, this reaction takes place through partial
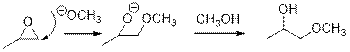
Epoxides are reactive, methoxide ion attacks epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product.
(c)
Interpretation:
The reason should be explained for the formation of six member ring compound in the given reaction.
Concept introduction:

In the presence of acid catalyst, this reaction takes place through partial
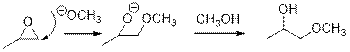
Epoxides are reactive, methoxide ion attacks epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
- Predict the following products. Then show the mechanism. H₂N NH2arrow_forwardBF3, Boron Trifluoride, known to contain three covalent boron-fluorine bonds. suggest and illustrate all of the processes as well as their energetical consequences for the formation of BF3 from its elements.arrow_forwardDraw the mechanism of the reaction.arrow_forward
- 9. Draw all of the possible Monochlorination Products that would Result From the Free Radical Chlormation OF 23,4-TRIMethyl Pentane b. Calculate the To Yield For the major • Product given the Following Relative Restritus For 1° 2° and 30 Hydrogens toward Free Radical Chloration 5.0: 38 : 1 30 2° 1° C. what would be the major product in the Free Radical brominator Of the Same Molecule. Explain your Reasoning.arrow_forwardWhat is the complete reaction mechanism for the chlorination of Ethane, C2H6?arrow_forwardA 13C NMR spectrum is shown for a molecule with the molecular formula of C6H100. Draw the structure that best fits this data. 220 200 180 160 140 120100 80 60 40 20 Drawingarrow_forward
- Based on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10. Provide assignment for the provided structurearrow_forwardO Predict the 'H NMR integration ratio for the following structure. IV I. 3 H A II. 1 H III. 2 H IV. 3 H I. 3 H B II. O H III. 2 H IV. 3 H I. 3 H C II. 2 H III. 2 Harrow_forward205. From the definition of the Gibbs free energy, G = H - TS, derive the Gibbs-Helmholtz equation a (or (G)),- =- H T2arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
