
Pearson eText Organic Chemistry -- Instant Access (Pearson+)
8th Edition
ISBN: 9780135213711
Author: Paula Bruice
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 10, Problem 68P
Interpretation Introduction
Interpretation:
The reason should be explained when (S)-2-butanol is heated with sulfuric acid gives racemic mixture.
Concept introduction:
Racemization:
Alcohol is treated with strong acid like sulfuric acid which forms stable carbocation and it can undergoes addition reaction which forms the racemic mixture.
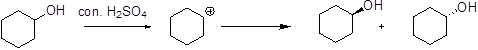
The stability of carbocation is given below,
Tertiary carbocation is more stable than the secondary and primary.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
>
You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other
major side products:
1. ☑
CI
2. H3O+
O
Draw the missing reagent X you think will make this synthesis work in the drawing area below.
If there is no reagent that will make your desired product in good yield or without complications, just check the box under the
drawing area and leave it blank.
Click and drag to start drawing a
structure.
Explanation
Check
?
DO
18
Ar
B
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Don't use ai to answer I will report you answer
Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence point
Chapter 10 Solutions
Pearson eText Organic Chemistry -- Instant Access (Pearson+)
Ch. 10.1 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 10.1 - Explain the difference in reactivity between...Ch. 10.1 - Prob. 5PCh. 10.1 - Prob. 6PCh. 10.1 - Prob. 8PCh. 10.2 - Prob. 9PCh. 10.3 - Prob. 11PCh. 10.3 - Show how 1-propanol can be converted into the...Ch. 10.4 - Which of the following alcohols dehydrates the...Ch. 10.4 - Prob. 14P
Ch. 10.4 - Prob. 15PCh. 10.4 - Propose a mechanism for each of the following...Ch. 10.4 - Draw the product of each of the following...Ch. 10.4 - Explain why the following alcohols, when heated...Ch. 10.4 - What stereoisomers are formed in the following...Ch. 10.4 - Prob. 20PCh. 10.4 - What alcohol would you treat with phosphorus...Ch. 10.5 - Prob. 22PCh. 10.6 - What are the major products obtained when each of...Ch. 10.6 - Prob. 26PCh. 10.7 - Prob. 27PCh. 10.7 - Would you expect the reactivity of a five-membered...Ch. 10.7 - Prob. 29PCh. 10.7 - What products are obtained from the reaction of...Ch. 10.7 - Prob. 31PCh. 10.7 - Prob. 32PCh. 10.7 - Prob. 33PCh. 10.8 - Draw the mechanism for formation of the two...Ch. 10.8 - Prob. 35PCh. 10.8 - Prob. 36PCh. 10.8 - How do the major products obtained from...Ch. 10.8 - Explain why the two arene oxides in Problem 38...Ch. 10.8 - Which compound is more likely to be carcinogenic?Ch. 10.8 - Three arene oxides can be obtained from...Ch. 10.9 - Explain why the half-life (the time it takes for...Ch. 10.10 - Prob. 43PCh. 10.10 - Prob. 44PCh. 10.10 - Prob. 45PCh. 10.10 - Prob. 46PCh. 10.10 - Prob. 47PCh. 10.10 - Describe a synthesis for each of the following...Ch. 10.11 - Using an alkyl halide and a thiol as starting...Ch. 10.11 - The following three nitrogen mustards were studied...Ch. 10.11 - Why is melphalan a good cancer drug?Ch. 10.11 - Prob. 53PCh. 10.12 - Propose a mechanism for the following reaction:Ch. 10 - Prob. 55PCh. 10 - Which compound is more likely to be carcinogenic?Ch. 10 - Prob. 57PCh. 10 - Prob. 58PCh. 10 - When heated with H2SO4, both...Ch. 10 - What is the major product obtained from the...Ch. 10 - Write the appropriate reagent over each arrow.Ch. 10 - What alkenes would you expect to be obtained from...Ch. 10 - Prob. 63PCh. 10 - Prob. 64PCh. 10 - When deuterated phenanthrene oxide undergoes a...Ch. 10 - An unknown alcohol with a molecular formula of...Ch. 10 - Explain why the acid-catalyzed dehydration of an...Ch. 10 - Prob. 68PCh. 10 - Prob. 69PCh. 10 - Propose a mechanism for the following reaction:Ch. 10 - What product would be formed if the four-membered...Ch. 10 - Which of the following ethers would be obtained in...Ch. 10 - Using the given starting material any necessary...Ch. 10 - Prob. 74PCh. 10 - When 3-methyl-2-butanol is heated with...Ch. 10 - Draw structures for compounds AF.Ch. 10 - Propose a mechanism for each of the following...Ch. 10 - How could you synthesize isopropyl propyl ether,...Ch. 10 - When ethyl ether is heated with excess HI for...Ch. 10 - When the following seven-membered ring alcohol is...Ch. 10 - Ethylene oxide reacts readily with HO because of...Ch. 10 - Describe how each of the following compounds could...Ch. 10 - Propose a mechanism for each of the following...Ch. 10 - Triethylene glycol is one of the products obtained...Ch. 10 - Prob. 85PCh. 10 - Propose a mechanism for the following reaction:Ch. 10 - Prob. 87PCh. 10 - An ion with a positively charged nitrogen atom in...Ch. 10 - The following reaction takes place several times...Ch. 10 - Prob. 90PCh. 10 - Propose a mechanism for each of the following...Ch. 10 - A vicinal diol has OH groups on adjacent carbons....Ch. 10 - Prob. 93PCh. 10 - Prob. 94PCh. 10 - Two stereoisomers are obtained from the reaction...Ch. 10 - Propose a mechanism for each or the following...Ch. 10 - Triethylenemelamine (TEM) is an antitumor agent....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning