
(a)
Interpretation:
The major product of given reaction should be given.
Concept introduction:
In the nucleophilic substitution reaction, the
In
The rate determination step is formation of carbocation.
The stability order of carbocation is,
Tertiary > Secondary > Primary
Therefore, tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation. Primary alcohol is less stable therefore it won’t undergoes
(b)
Interpretation:
The major product of given reaction should be given.
Concept introduction:
In the nucleophilic substitution reaction, the rate of reaction depends on reactant as well as nucleophile, which are involved in reaction is called bimolecular nucleophilic substitution reaction.
In
Reactant and nucleophile are present at the rate determination step.
The order of species involving in
Tertiary < Secondary < Primary
(c)
Interpretation:
The major product of given reaction should be given.
Concept introduction:
Chromic Acid:
Chromic Acid (
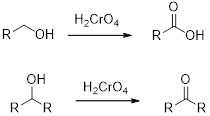
(d)
Interpretation:
The major product of given reaction should be given.
Concept introduction:
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid.
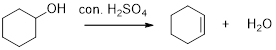
The stability of carbocation is given below,
Tertiary carbocation is more stable than the secondary and primary.
(e)
Interpretation:
The major product of given reaction should be given.
Concept introduction:
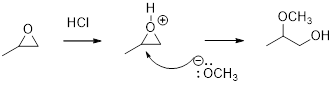
In the presence of acid catalyst, this reaction takes place through partial SN1 and partial SN2 pathway.
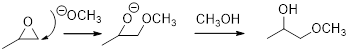
Epoxides are reactive, methoxide ion attacks the Epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product.
(f)
Interpretation:
The major product of given reaction should be given.
Concept introduction:

In the presence of acid catalyst, this reaction takes place through partial SN1 and partial SN2 pathway. Epoxides are reactive, Epoxides get protonated followed by alcohol attacks to the stable carbocation and form the product.
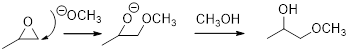
Epoxides are reactive, methoxide ion attacks the Epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product.
(g)
Interpretation:
The major product should be identified.
Concept introduction:
Tosylation reaction:
The alcohol is treated with any tosyl chloride (methane sulfonyl chloride) which yields tosylated product this reaction is called as alkyl tosylate and which is shown below,
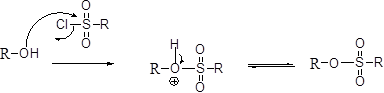
The alcohols is reaction with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms which is bearing alcohol group which yield the corresponding inversion product.
(h)
Interpretation:
The major product of given reaction should be given.
Concept introduction:
Oxidation of alcohol:
Alcohols reaction with hypochlorous (oxidizing agent) in the presence of acetic acid which yields the corresponding
Primary alcohols gives aldehyde, secondary alcohols gives ketone.
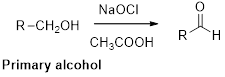
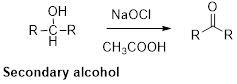
(i)
Interpretation:
The major product should be identified.
Concept introduction:
Generally
For elimination reaction quartnary ammonium halide can be converted in to quartnary ammonium hydroxide by using aqueous silver oxide.
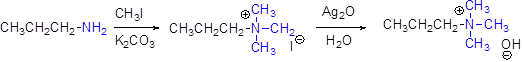
Hofmann elimination:
Quartnary ammonium ion undergoes elimination when using strong base like hydroxide ion this reaction is called as hofmann elimination. Proton abstraction is takes place in β- carbon atom which is having more number of hydrogen.

(j)
Interpretation:
The major product should be identified.
Concept introduction:
The alcohols is reaction with acids like hydrochloric acid or hydrobromic which yield the corresponding carbocation intermediate, this carbocation intermediate undergoes substitution reaction which yields the corresponding substitution product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
Primary alcohol is less stable therefore it won’t undergoes
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Pearson eText Organic Chemistry -- Instant Access (Pearson+)
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





