
Concept explainers
Which pair of elements has the most similar Lewis structures?
a. N and S
b. F and Ar
c. Cl and Ar
d. O and S
Interpretation: The pair of elements that would have the most similar structure is to be determined.
Concept Introduction:
Lewis structure is used to represent the molecules. It is also known as dot structure. In Lewis model, valence electrons are represented as dots.
Answer to Problem 1SAQ
Correct Answer: The most similar Lewis structure is of
Therefore, the correct answer is option (d).
Explanation of Solution
Reason for correct option:
Oxygen
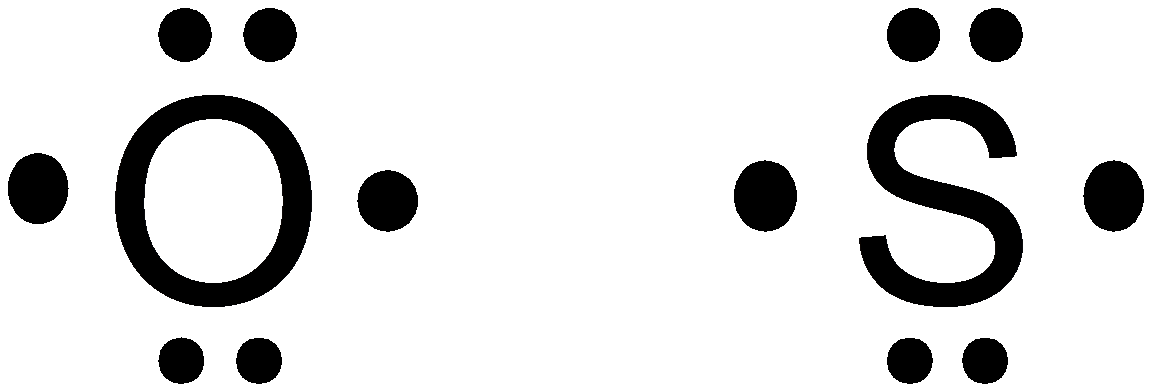
Thus,
Hence, option (d) is correct.
Reasons for incorrect options:
Option (a) is given as
Nitrogen falls in group
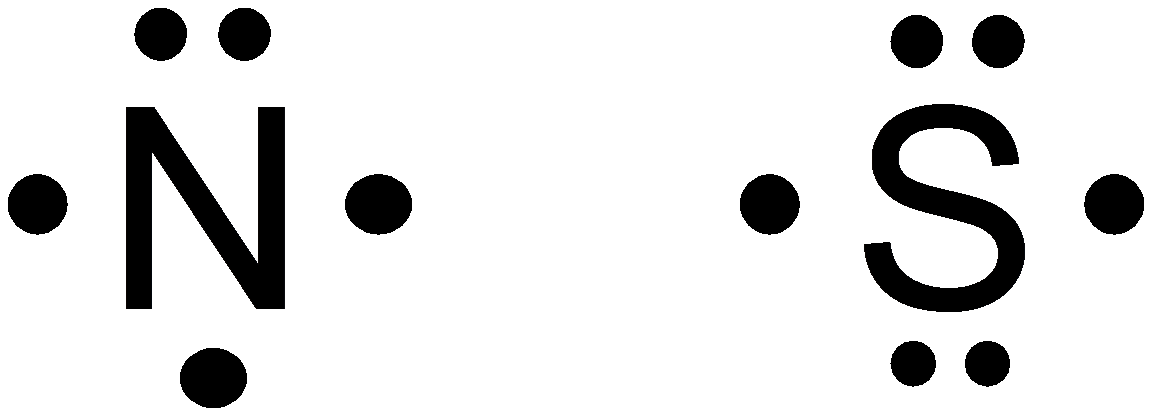
So, it is a wrong answer.
Option (b) is given as
Fluorine falls in
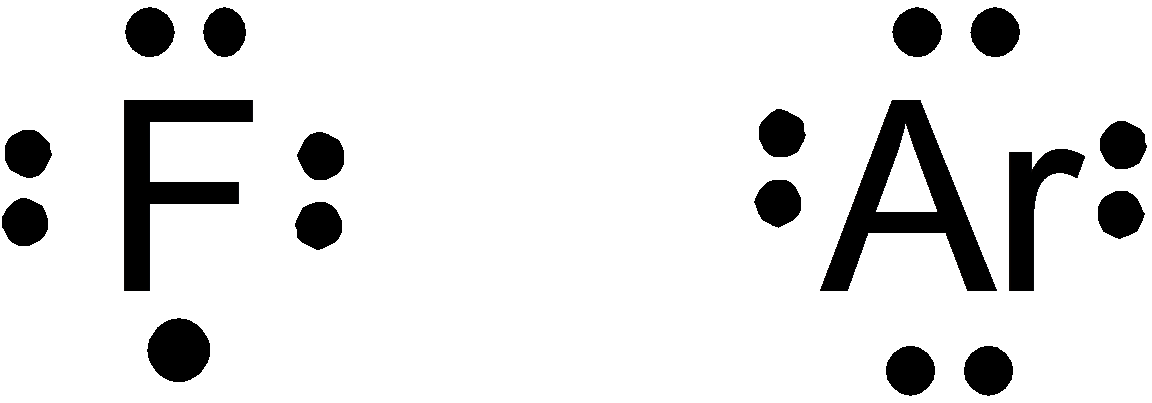
So, it is a wrong answer.
Option (c) is given as
Chlorine falls in group

So, it is a wrong answer.
Hence, options (a), (b) and (c) are incorrect.
Want to see more full solutions like this?
Chapter 10 Solutions
Introductory Chemistry (5th Edition) (Standalone Book)
- The number of carbon skeletons that have 8 carbons, one of which istertiary is A. 7; B. More than 7; C. 6; D. 5; E. 4arrow_forwardThe azide ion is N3^-. In addition to the ionic charge, it’s three mostimportant contributing structures also have formal charges. The totalnumber of π bonds in these three contributing structures isA. 6; B. 12; C. 3; D. 9; E. None of the other answers is correct.arrow_forwardThe sum of the numerals in the name of the compoundis A. None of the other answers is correct.; B. 11;C. 6; D. 8; E. 5.arrow_forward
- A compound has a six carbon ring with three double bonds. Attachedto the ring is a three carbon chain with a triple bond and a two carbonchain with two bromines attached. The number of hydrogens in a molecule of this compound is A. 10; B. 12; C. 14; D. 13; E. None of the other answers is correct.arrow_forwardCan you help me? I can't seem to understand the handwriting for the five problems, and I want to be able to solve them and practice. If you'd like to give me steps, please do so to make it easier understand.arrow_forwardThe number of 2sp3 hybrid orbitals in the moleculeis A. 12; B. 8; C. 3; D. 11; E. None of the other answers is correct.arrow_forward

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





