
Concept explainers
Interpretation:
The molecular geometry of each of the given molecules is to be determined.
Concept Introduction: While writing the Lewis structure of a covalent molecule these steps are followed:
First, the correct skeleton is written for the molecule
Total number of valence electrons are calculated
Distribution of a total number of valence electrons in such a way that it completes the octet configuration of all the constituent atoms.
If any atom lacks the octet configuration the double or triple bonds are added.
For molecules which obey the octet rule must have eight electrons in their valence shell. In the molecule, the total number of electron groups around the central atom is equal to the sum of lone pairs, a single bond, a double bond and triple bond around the central atom.
Valence shell electron pair repulsion (VSEPR) theory and Lewis model helps in determining the shape of the molecule.
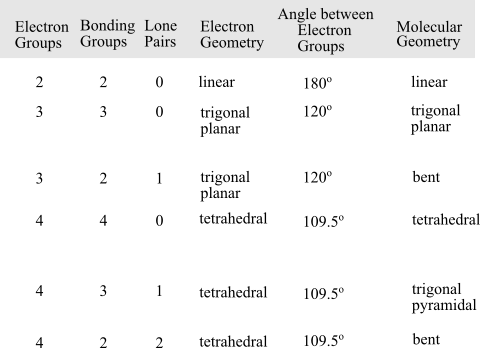
The molecular geometry of a molecule can be determined by consider the total number of electron groups, bonding groups and the lone pairs. On the basis of electron groups, the molecular geometry is predicted. To determine the geometry of the molecule, follow the following table:
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Introductory Chemistry (5th Edition) (Standalone Book)
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





