
Concept explainers
(a)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
Oxidation of alcohol is the process in which the hydroxyl group of alcohol gets transformed either into carboxyl group or into an
(a)
Explanation of Solution
The given blank is: Can be oxidized to an aldehyde _______
Oxidation of alcohol is the process in which the hydroxyl group of alcohol gets transformed either into carboxyl group or into an aldehyde or to ketone. Product obtained may depend on the oxidizing agent used. Therefore, in presence of mild oxidizing agent, one gets aldehyde or ketone. In the presence of strong oxidizing agent,
Thus, Can be oxidized to an aldehyde:
(b)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
(b)
Explanation of Solution
The given blank is: Segment of a condensation polymer molecule _______.
The
Thus, segment of a condensation polymer molecule
(c)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
Oxidation of alcohol is the process in which the hydroxyl group of alcohol gets transformed either into carboxyl group or into an aldehyde or to ketone. Product obtained may depend on the oxidizing agent used. Therefore, in presence of mild oxidizing agent, one gets aldehyde or ketone.
(c)
Explanation of Solution
The given blank is: Can be oxidized to a carboxylic acid _____.
Oxidation of alcohol is the process in which the hydroxyl group of alcohol gets transformed either into carboxyl group or into an aldehyde or to ketone. Product obtained may depend on the oxidizing agent used. Therefore, in presence of mild oxidizing agent, one gets aldehyde or ketone. In the presence of strong oxidizing agent, carboxylic acid is obtained.
Thus, Can be oxidized to a carboxylic acid
(d)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
There are three types of alcohols: primary, secondary and tertiary. The type of alcohols depends upon the carbon atom to which the hydroxyl group is attached.
(d)
Explanation of Solution
The given blank is: A primary alcohol_____.
The compound given in Boxes A and G is a primary alcohol. The hydroxyl group is attached with a carbon atom that is attached with one carbon atom.
Thus, a primary alcohol
(e)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
The fatty acid can react with glycerol to form triglyceride with the formation of ester linkages. The reaction between acid and alcohol gives an ester as a major product. The ester group is derived by a carboxylic acid group in which the acidic hydrogen is replaced by an alkyl group.
(e)
Explanation of Solution
The given blank is: A triglyceride_____.
The triglyceride is formed by the reaction of glycerol and fatty acids. It is characterized by the presence of ester linkages. Box F contains a triglyceride.
Thus, A triglyceride
(f)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
Addition polymerization is the polymerization in which monomer units are linked together with no side product formation. Addition polymerization is given by the molecules which contains carbon-carbon double bond.
(f)
Explanation of Solution
The given blank is: Addition polymerization monomer_____.
Addition polymerization is given by the molecules which contains carbon-carbon double bond. Compounds in Box C contain a double bond.
Thus, addition polymerization monomer is
(g)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
An amide bond is formed between a carboxylic acid and an
(g)
Explanation of Solution
The given blank is: Contains an amide linkage_____.
An amide linkageis formed between a carboxylic acid and an amine. The carboxylic acid group of one amino acid reacts with an amino group of another amino acid to form an amide linkageby removing a water molecule. Compound in Box D and Box H contains an amide bond.
Thus, contains an amide linkage
(h)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
Oxidation of alcohol is the process in which the hydroxyl group of alcohol gets transformed either into an aldehyde or into ketone. To get an aldehyde or ketone, oxidation of acohol must takes place in the presence of mild oxidizing agent such as copper at 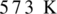 . Product obtained may be aldehyde or ketone. It depends on the nature of alcohol which may be primary, or secondary alcohol.
. Product obtained may be aldehyde or ketone. It depends on the nature of alcohol which may be primary, or secondary alcohol.
(h)
Explanation of Solution
The given blank is: Oxidized to form a ketone_____.
Oxidation of alcohol is the process in which the hydroxyl group of alcohol gets transformed either into an aldehyde or into ketone. Secondary alcohols undergo oxidation to form ketones. Compound in Box E and Box I are the secondary alcohols.
Thus, oxidized to form a ketone
(i)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
The reaction between acid and alcohol gives an ester as a major product. The ester is derived by a carboxylic acid group in which the acidic hydrogen is replaced by an alkyl group.
(i)
Explanation of Solution
The given blank is: Contains an ester linkage_____.
The triglyceride is formed by the reaction of glycerol and fatty acids. It is characterized by the presence of ester linkages. Box F contains a triglyceride.
Thus, contains an ester linkage
(j)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
Polymers are the high molecular mass compounds. The small monomers units joined together to form large molecule and this reaction is known as polymerization reaction. When two small molecules condense together to form a polymer with elimination of water molecule, the reaction is known as condensation polymerization.
(j)
Explanation of Solution
The given blank is: Condensation polymerization monomer_____.
Box B contains a compound in which two amine groupsare present. These two amine groups can undergo condensation polymerization which is characterized by the loss of hydrogen molecule.
Thus, Condensation polymerization monomer
Want to see more full solutions like this?
Chapter 10 Solutions
OWLV2 FOR MOORE/STANITSKI'S CHEMISTRY:
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardHelp me solve this problem. Thank you in advance.arrow_forward22.7 Predict the monoalkylated products of the following reactions with benzene. (a) AlCl3 Ya (b) AlCl3 (c) H3PO4 (d) 22.8 Think-Pair-Share AICI3 The reaction below is a common electrophilic aromatic substitution. SO3 H₂SO4 SO₂H (a) Draw the reaction mechanism for this reaction using HSO,+ as the electrophile. (b) Sketch the reaction coordinate diagram, where the product is lower in energy than the starting reactant. (c) Which step in the reaction mechanism is highest in energy? Explain. (d) Which of the following reaction conditions could be used in an electrophilic aro- matic substitution with benzene to provide substituted phenyl derivatives? (i) AICI3 HNO3 H₂SO4 K2Cr2O7 (iii) H₂SO4 (iv) H₂PO₁arrow_forward
- Is an acid-base reaction the only type of reaction that would cause leavening products to rise?arrow_forwardHelp me understand this! Thank you in advance.arrow_forward22.22 For each compound, indicate which group on the ring is more strongly activating and then draw a structural formula of the major product formed by nitration of the compound. Br CHO (a) CH3 (b) (c) CHO CH3 SO₂H (d) ☑ OCHS NO₂ (e) (f) CO₂H NHCOCH3 NHCOCH, (h) CHS 22.23 The following molecules each contain two aromatic rings. (b) 000-100- H3C (a) (c) Which ring in each undergoes electrophilic aromatic substitution more readily? Draw the major product formed on nitration.arrow_forward
- V Consider this step in a radical reaction: Br: ? What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ⚫ionization termination initialization neutralization none of the abc Explanation Check 80 Ο F3 F1 F2 2 F4 01 % do5 $ 94 #3 X 5 C MacBook Air 25 F5 F6 66 ©2025 ˇ F7 29 & 7 8arrow_forwardShow how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forward
- Polymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forwardDraw a tetramer if this alternating copolymer pleasearrow_forwardDraw the monomers required to synthesize this condensation polymer.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





