
Chemistry
13th Edition
ISBN: 9781259911156
Author: Raymond Chang Dr., Jason Overby Professor
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.93QP
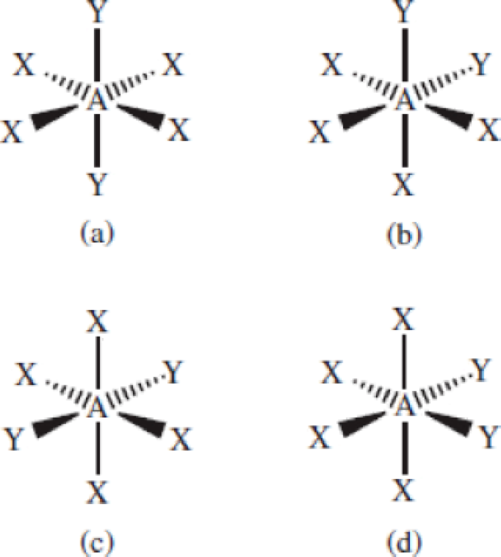
The following molecules (AX4Y2) all have octahedral geometry. Group the molecules that are equivalent to each other.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Add conditions above and below the arrow that turn the reactant below into the product below in a single transformation.
+ More...
If you need to write reagents above and below the arrow that have complex hydrocarbon groups in them, there is a set of standard abbreviations you can use.
More...
T
H,N
NC
Dat
Indicate the order of basicity of primary, secondary and tertiary amines.
>
Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic.
Cl
Z-
N
O aromatic
O antiaromatic
O nonaromatic
O aromatic
O antiaromatic
O nonaromatic
O aromatic
○ antiaromatic
nonaromatic
Chapter 10 Solutions
Chemistry
Ch. 10.1 - Use the VSEPR model to predict the geometry of (a)...Ch. 10.1 - What is the molecular geometry of GeCl4?Ch. 10.1 - What is the molecular geometry of BrO3?Ch. 10.1 - Which of the following geometries has a greater...Ch. 10.2 - Does the AlCl3 molecule have a dipole moment?Ch. 10.2 - Predict whether PF5 has a dipole moment.Ch. 10.2 - The molecule CF4 does not have a dipole moment...Ch. 10.2 - Carbon dioxide has a linear geometry and is...Ch. 10.3 - Compare the Lewis theory and the valence bond...Ch. 10.4 - Determine the hybridization state of the...
Ch. 10.4 - Describe the hybridization state of Se in SeF6.Ch. 10.4 - How many orbitals does a set of sp3d hybrid...Ch. 10.4 - What is the hybridization of P in PH4+?Ch. 10.4 - What is the hybridization of Xe in XeF4Ch. 10.5 - Describe the bonding in the hydrogen cyanide...Ch. 10.5 - How many pi bonds are present in CS2?Ch. 10.5 - Which of the following pairs of atomic orbitals on...Ch. 10.6 - One way to account for the fact that an O2...Ch. 10.7 - Which of the following species has a longer bond...Ch. 10.7 - Calculate the bond order of F2+.Ch. 10.7 - Determine if N2+ is diamagnetic or paramagnetic.Ch. 10.7 - Estimate the bond enthalpy (kJ/mol) of the H2+...Ch. 10.8 - Describe the bonding in the nitrate ion (NO3) in...Ch. 10 - How is the geometry of a molecule defined and why...Ch. 10 - Sketch the shape of a linear triatomic molecule, a...Ch. 10 - How many atoms are directly bonded to the central...Ch. 10 - Discuss the basic features of the VSEPR model....Ch. 10 - Prob. 10.5QPCh. 10 - Prob. 10.6QPCh. 10 - Predict the geometries of the following species...Ch. 10 - Predict the geometries of the following species:...Ch. 10 - Predict the geometry of the following molecules...Ch. 10 - Predict the geometry of the following molecules...Ch. 10 - Predict the geometry of the following molecules...Ch. 10 - Predict the geometries of the following ions: (a)...Ch. 10 - Describe the geometry around each of the three...Ch. 10 - Which of the following species are tetrahedral?...Ch. 10 - Prob. 10.15QPCh. 10 - Prob. 10.16QPCh. 10 - Prob. 10.17QPCh. 10 - The bonds in beryllium hydride (BeH2) molecules...Ch. 10 - Referring to Table 10.3, arrange the following...Ch. 10 - The dipole moments of the hydrogen halides...Ch. 10 - List the following molecules in order of...Ch. 10 - Does the molecule OCS have a higher or lower...Ch. 10 - Which of the molecules (a) or (b) has a higher...Ch. 10 - Prob. 10.24QPCh. 10 - What is valence bond theory? How does it differ...Ch. 10 - Use valence bond theory to explain the bonding in...Ch. 10 - Prob. 10.27QPCh. 10 - Prob. 10.28QPCh. 10 - Prob. 10.29QPCh. 10 - What is the angle between the following two hybrid...Ch. 10 - Describe the bonding scheme of the AsH3 molecule...Ch. 10 - What is the hybridization state of Si in SiH4 and...Ch. 10 - Describe the change in hybridization (if any) of...Ch. 10 - Consider the reaction BF3+NH3F3BNH3 Describe the...Ch. 10 - What hybrid orbitals are used by nitrogen atoms in...Ch. 10 - Prob. 10.36QPCh. 10 - Give the formula of a cation comprised of iodine...Ch. 10 - Give the formula of an anion comprised of iodine...Ch. 10 - How would you distinguish between a sigma bond and...Ch. 10 - What are the hybrid orbitals of the carbon atoms...Ch. 10 - Specify which hybrid orbitals are used by carbon...Ch. 10 - Prob. 10.42QPCh. 10 - The allene molecule H2CCCH2 is linear (the three C...Ch. 10 - How many pi bonds and sigma bonds are there in the...Ch. 10 - How many sigma bonds and pi bonds are there in...Ch. 10 - What is molecular orbital theory? How does it...Ch. 10 - Sketch the shapes of the following molecular...Ch. 10 - Explain the significance of bond order. Can bond...Ch. 10 - Explain in molecular orbital terms the changes in...Ch. 10 - The formation of H2 from two H atoms is an...Ch. 10 - Prob. 10.51QPCh. 10 - Arrange the following species in order of...Ch. 10 - Prob. 10.53QPCh. 10 - Which of these species has a longer bond, B2 or...Ch. 10 - Acetylene (C2H2) has a tendency to lose two...Ch. 10 - Compare the Lewis and molecular orbital treatments...Ch. 10 - Explain why the bond order of N2 is greater than...Ch. 10 - Compare the relative stability of the following...Ch. 10 - Use molecular orbital theory to compare the...Ch. 10 - A single bond is almost always a sigma bond, and a...Ch. 10 - In 2009 the ion N23 was isolated. Use a molecular...Ch. 10 - The following potential energy curve represents...Ch. 10 - Prob. 10.63QPCh. 10 - Prob. 10.64QPCh. 10 - Prob. 10.65QPCh. 10 - Explain why the symbol on the left is a better...Ch. 10 - Determine which of these molecules has a more...Ch. 10 - Nitryl fluoride (FNO2) is very reactive...Ch. 10 - Describe the bonding in the nitrate ion NO3 in...Ch. 10 - Prob. 10.70QPCh. 10 - Which of the following species is not likely to...Ch. 10 - Draw the Lewis structure of mercury(II) bromide....Ch. 10 - Sketch the bond moments and resultant dipole...Ch. 10 - Although both carbon and silicon are in Group 4A,...Ch. 10 - Acetaminophen is the active ingredient in Tylenol....Ch. 10 - Caffeine is a stimulant drug present in coffee....Ch. 10 - Predict the geometry of sulfur dichloride (SCl2)...Ch. 10 - Antimony pentafluoride, SbF5, reacts with XeF4 and...Ch. 10 - Draw Lewis structures and give the other...Ch. 10 - Predict the bond angles for the following...Ch. 10 - Briefly compare the VSEPR and hybridization...Ch. 10 - Describe the hybridization state of arsenic in...Ch. 10 - Draw Lewis structures and give the other...Ch. 10 - Which of the following molecules and ions are...Ch. 10 - Prob. 10.85QPCh. 10 - The N2F2 molecule can exist in either of the...Ch. 10 - Cyclopropane (C3H6) has the shape of a triangle in...Ch. 10 - The compound 1,2-dichloroethane (C2H4Cl2) is...Ch. 10 - Does the following molecule have a dipole moment?...Ch. 10 - So-called greenhouse gases, which contribute to...Ch. 10 - The bond angle of SO2 is very close to 120, even...Ch. 10 - 3-azido-3-deoxythymidine, shown here, commonly...Ch. 10 - The following molecules (AX4Y2) all have...Ch. 10 - The compounds carbon tetrachloride (CCl4) and...Ch. 10 - Prob. 10.95QPCh. 10 - What are the hybridization states of the C and N...Ch. 10 - Use molecular orbital theory to explain the...Ch. 10 - Referring to the Chemistry in Action essay...Ch. 10 - Which of the molecules (a)(c) are polar?Ch. 10 - Prob. 10.100QPCh. 10 - The stable allotropic form of phosphorus is P4, in...Ch. 10 - Referring to Table 9.4, explain why the bond...Ch. 10 - Use molecular orbital theory to explain the...Ch. 10 - The ionic character of the bond in a diatomic...Ch. 10 - Prob. 10.105QPCh. 10 - Prob. 10.106QPCh. 10 - Aluminum trichloride (AlCl3) is an...Ch. 10 - The molecules cis-dichloroethylene and...Ch. 10 - Prob. 10.109QPCh. 10 - Prob. 10.110QPCh. 10 - The molecule benzyne (C6H4) is a very reactive...Ch. 10 - Assume that the third-period element phosphorus...Ch. 10 - Consider a N2 molecule in its first excited...Ch. 10 - Prob. 10.114QPCh. 10 - Prob. 10.116QPCh. 10 - Draw the Lewis structure of ketene (C2H2O) and...Ch. 10 - TCDD, or 2,3,7,8-tetrachlorodibenzo-p-dioxin, is a...Ch. 10 - Write the electron configuration of the cyanide...Ch. 10 - Prob. 10.120QPCh. 10 - The geometries discussed in this chapter all lend...Ch. 10 - Prob. 10.122QPCh. 10 - Which of the following ions possess a dipole...Ch. 10 - Given that the order of molecular orbitals for NO...Ch. 10 - Shown here are molecular models of SX4 for X = F,...Ch. 10 - Based on what you have learned from this chapter...Ch. 10 - How many carbon atoms are contained in one square...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me answer this question. I don't understand how or even if this can happen in a single transformation. Please provide a detailed explanation and a drawing showing how it can happen in a single transformation. Add the necessary reagents and reaction conditions above and below the arrow in this organic reaction. If the products can't be made from the reactant with a single transformation, check the box under the drawing area instead.arrow_forward2) Draw the correct chemical structure (using line-angle drawings / "line structures") from their given IUPAC name: a. (E)-1-chloro-3,4,5-trimethylhex-2-ene b. (Z)-4,5,7-trimethyloct-4-en-2-ol C. (2E,6Z)-4-methylocta-2,6-dienearrow_forwardපිපිම Draw curved arrows to represent the flow of electrons in the reaction on the left Label the reactants on the left as either "Acid" or "Base" (iii) Decide which direction the equilibrium arrows will point in each reaction, based on the given pk, values (a) + H-O H 3-H + (c) H" H + H****H 000 44-00 NH₂ (e) i Дон OH Ө NHarrow_forward
- 3) Label the configuration in each of the following alkenes as E, Z, or N/A (for non-stereogenic centers). 00 E 000 N/A E Br N/A N/A (g) E N/A OH E (b) Oz N/A Br (d) 00 E Z N/A E (f) Oz N/A E (h) Z N/Aarrow_forward6) Fill in the missing Acid, pKa value, or conjugate base in the table below: Acid HCI Approximate pK, -7 Conjugate Base H-C: Hydronium (H₂O') -1.75 H-O-H Carboxylic Acids (RCOOH) Ammonium (NH4) 9.24 Water (H₂O) H-O-H Alcohols (ROH) RO-H Alkynes R--H Amines 25 25 38 HOarrow_forward5) Rank the following sets of compounds in order of decreasing acidity (most acidic to least acidic), and choose the justification(s) for each ranking. (a) OH V SH я вон CH most acidic (lowst pKa) least acidic (highest pKa) Effect(s) Effect(s) Effect(s) inductive effect O inductive effect O inductive effect electronegativity electronegativity O electronegativity resonance polarizability resonance polarizability O resonance O polarizability hybridization Ohybridization O hybridization оarrow_forward
- How negatively charged organic bases are formed.arrow_forwardNonearrow_forward1) For the following molecules: (i) Label the indicated alkenes as either cis (Z), trans (E), or N/A (for non-stereogenic centers) by bubbling in the appropriate label on the molecule. (ii) Complete the IUPAC name located below the structure (HINT: Put the letter of the configuration in parentheses at the beginning of the name!) E z N/A ()-3,4,6-trimethylhept-2-ene E Oz O N/A ()-3-ethyl-1-fluoro-4-methylhex-3-ene E -+- N/A Me )-2,3-dimethylpent-2-ene (d) (b) E O N/A Br ()-5-bromo-1-chloro-3-ethyloct-4-ene ОЕ Z N/A Et (___)-3-ethyl-4-methylhex-3-ene E (f) Oz N/A z N/A HO (4.7)-4-(2-hydroxyethyl)-7-methylnona-4,7-dien-2-onearrow_forward
- O 9:21AM Tue Mar 4 ## 64% Problem 51 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H :0: CI. AI :CI: :CI: Cl AI Select to Add Arrows Select to Add Arrows O: Cl :CI: :0: H CI: CI CO Select to Add Arrows Select to Add Arrows :O: CI :0: Cl. 10: AIarrow_forward(i) Draw in the missing lone pair(s) of electrons of the reactants on the left (ii) Draw (curved) arrows to show the flow of electrons in the acid/base reaction on the left (iii) Draw the products of the acid/base on the right (iv) Select the correct label for each product as either "conjugate acid" or "conjugate base" (a) JOH OH NH₂ acid base (b) De "H conjugate acid conjugate acid conjugate base conjugate base acid base conjugate acid conjugate base conjugate acid conjugate base acid basearrow_forwardCould someone answer this NMR and explain please Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided below.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY