
Concept explainers
Interpretation:
The closest cubic cell where crystallization of Titanium takes places has to be identified.
Concept Introduction:
Body-centered unit cell:
A body – centered cubic cell is a type of unit cell with atoms arranged in all the vertices of the unit cell and one atom at the center of the cube.
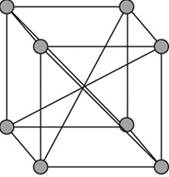
Figure 1
A body – centered cubic unit cell has its all the eight vertices arranged with atoms in it. Eight atoms are arranged in a simple cubic unit cell in eight vertices. Each atom in a vertex is shared by eight neighboring unit cells such that each cubic unit cell has
Face-centered cubic:
In face-centered cubic unit cell, each of the six corners is occupied by every single atom. Each face of the cube is occupied by one atom.
Each atom in the corner is shared by eight unit cells and each atom in the face is shared by two unit cells. Thus the number of atoms per unit cell in FCC unit cell is,
Simple cubic unit:
A simple cubic unit cell is the simplest form of a cubic unit cell. A cube has eight vertices, twelve edges and six faces. Similarly a cubic unit cell has eight vertices, twelve edges and six faces. If in a cubic unit cell, the components occupy only the eight vertices, then the unit cell is known as simple cubic unit cell.
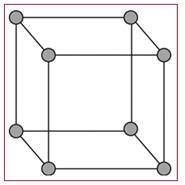
Figure 2
A simple cubic unit cell has its all the eight vertices arranged with atoms in it. Thus eight atoms are arranged in a simple cubic unit cell in eight vertices. Each atom in a vertex is shared by eight neighboring unit cells. Each layer of a lattice has four unit cells and the upper or lower to that layer has four unit cells. So, each simple cubic unit cell has
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
General Chemistry: Atoms First
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forward
- Which of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forwardWhat is the major product of the following reaction? O O OH OH 1. BH 2. H₂O₂, NaOH OH OHarrow_forwardDraw the products formed when each ester is hydrolyzed with water and sulfuric acid.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





