
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
5th Edition
ISBN: 9780321967466
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 10, Problem 10.41UTC
Interpretation Introduction
Interpretation:
‘Value of equilibrium constant is larger or smaller’ should be identified.
Concept introduction:
Equilibrium is the condition at which the rate of formation of product is equal to rate of disappearance of reactant.
Equilibrium constant is the concentration of product raise to its molecular coefficient divided to the concentration of reactant raise to its molecular coefficient, pure solids and liquids are not included in the equilibrium constant expression. and shown as below:
Given:
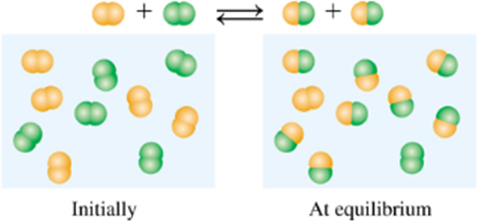
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the single step reaction: A + B → 2C + 25 kJ
If the activation energy for this reaction is 35.8 kJ, sketch an energy vs. reaction coordinate diagram for this reaction. Be sure to label the following on your diagram: each of the axes, reactant compounds and product compounds, enthalpy of reaction, activation energy of the forward reaction with the correct value, activation energy of the backwards reaction with the correct value and the transition state.
In the same sketch you drew, after the addition of a homogeneous catalyst, show how it would change the graph. Label any new line "catalyst" and label any new activation energy.
How many grams of C are combined with 3.75 ✕ 1023 atoms of H in the compound C5H12?
e.
f. CH3O.
יון
Br
NaOCH3
OCH 3
Br
H₂O
Chapter 10 Solutions
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Ch. 10.1 - What is meant by the rate of a reaction? Why does...Ch. 10.1 - How does a catalyst affect the activation energy?...Ch. 10.1 - Prob. 10.3QAPCh. 10.1 - Prob. 10.4QAPCh. 10.1 - Prob. 10.5QAPCh. 10.1 - Prob. 10.6QAPCh. 10.2 - Prob. 10.7QAPCh. 10.2 - Prob. 10.8QAPCh. 10.2 - Prob. 10.9QAPCh. 10.2 - Prob. 10.10QAP
Ch. 10.2 - Prob. 10.11QAPCh. 10.2 - Prob. 10.12QAPCh. 10.3 - Prob. 10.13QAPCh. 10.3 - Prob. 10.14QAPCh. 10.3 - Prob. 10.15QAPCh. 10.3 - Prob. 10.16QAPCh. 10.3 - Prob. 10.17QAPCh. 10.3 - Prob. 10.18QAPCh. 10.3 - Prob. 10.19QAPCh. 10.3 - Prob. 10.20QAPCh. 10.4 - Prob. 10.21QAPCh. 10.4 - Prob. 10.22QAPCh. 10.4 - Prob. 10.23QAPCh. 10.4 - Prob. 10.24QAPCh. 10.4 - Prob. 10.25QAPCh. 10.4 - Prob. 10.26QAPCh. 10.4 - Prob. 10.27QAPCh. 10.4 - The Kc for the following reaction at 225C is...Ch. 10.5 - Prob. 10.29QAPCh. 10.5 - Ammonia is produced by reacting nitrogen gas and...Ch. 10.5 - Prob. 10.31QAPCh. 10.5 - Prob. 10.32QAPCh. 10.5 - Prob. 10.33QAPCh. 10.5 - Prob. 10.34QAPCh. 10.5 - Prob. 10.35QAPCh. 10.5 - Prob. 10.36QAPCh. 10.5 - Prob. 10.37QAPCh. 10.5 - Prob. 10.38QAPCh. 10 - Prob. 10.39UTCCh. 10 - Prob. 10.40UTCCh. 10 - Prob. 10.41UTCCh. 10 - Prob. 10.42UTCCh. 10 - Prob. 10.43UTCCh. 10 - Prob. 10.44UTCCh. 10 - Prob. 10.45AQAPCh. 10 - For each of the following changes at equilibrium,...Ch. 10 - Prob. 10.47AQAPCh. 10 - Prob. 10.48AQAPCh. 10 - Consider the reaction: (10.3) 2NH3gN2g+3H2g a...Ch. 10 - Consider the reaction: (10.3) 2SO2g+O2g2SO3g a...Ch. 10 - Prob. 10.51AQAPCh. 10 - Prob. 10.52AQAPCh. 10 - According to Le Châtelier’s principle, does the...Ch. 10 - Prob. 10.54AQAPCh. 10 - Prob. 10.55AQAPCh. 10 - Prob. 10.56AQAPCh. 10 - Prob. 10.57CQCh. 10 - Prob. 10.58CQCh. 10 - Prob. 10.59CQCh. 10 - Prob. 10.60CQCh. 10 - Prob. 10.61CQCh. 10 - Prob. 10.62CQCh. 10 - Prob. 10.63CQCh. 10 - Indicate if you would increase or decrease the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward5. A solution of sucrose is fermented in a vessel until the evolution of CO2 ceases. Then, the product solution is analyzed and found to contain, 45% ethanol; 5% acetic acid; and 15% glycerin by weight. If the original charge is 500 kg, evaluate; e. The ratio of sucrose to water in the original charge (wt/wt). f. Moles of CO2 evolved. g. Maximum possible amount of ethanol that could be formed. h. Conversion efficiency. i. Per cent excess of excess reactant. Reactions: Inversion reaction: C12H22O11 + H2O →2C6H12O6 Fermentation reaction: C6H12O6 →→2C2H5OH + 2CO2 Formation of acetic acid and glycerin: C6H12O6 + C2H5OH + H₂O→ CH3COOH + 2C3H8O3arrow_forward
- Show work. don't give Ai generated solution. How many carbons and hydrogens are in the structure?arrow_forward13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B 2°C. +2°C. cleavage Bond A •CH3 + 26.← Cleavage 2°C. + Bond C +3°C• CH3 2C Cleavage E 2°C. 26. weakest bond Intact molecule Strongest 3°C 20. Gund Largest argest a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. C Weakest bond A Produces Most Bond Strongest Bond Strongest Gund produces least stable radicals Weakest Stable radical b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. 13°C. formed in bound C cleavage ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. • CH3 methyl radical Formed in Gund A Cleavage c.…arrow_forwardBr. COOH Br, FCH COOH E FeBr ASOCI B NH (CH,CO),OD Br₂ 2 C alcKOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY