
Chemistry Principles And Practice
3rd Edition
ISBN: 9781305295803
Author: David Reger; Scott Ball; Daniel Goode
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.106QE
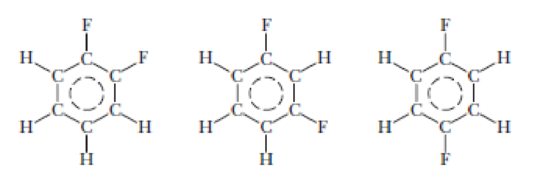
Following are the structures of three isomers of difluorobenzene, C6H4F2. Are any of them nonpolar?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate
the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data:
molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.
Determine the distance between the metal and the OHP layer using the Helm-
holtz model when the electrode's differential capacitance is 145 μF cm².
DATA: dielectric constant of the medium for the interfacial zone &r=
lectric constant of the vacuum &0 = 8.85-10-12 F m-1
= 50, die-
Describe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.
Chapter 10 Solutions
Chemistry Principles And Practice
Ch. 10 - Prob. 10.1QECh. 10 - Prob. 10.2QECh. 10 - Prob. 10.3QECh. 10 - Prob. 10.4QECh. 10 - Prob. 10.5QECh. 10 - Prob. 10.6QECh. 10 - Prob. 10.7QECh. 10 - Prob. 10.8QECh. 10 - Prob. 10.9QECh. 10 - Prob. 10.10QE
Ch. 10 - Which atomic orbitals overlap to form the bonds in...Ch. 10 - Prob. 10.12QECh. 10 - Identify the hybrid orbitals used by boron in BCl3...Ch. 10 - Identify the hybrid orbitals used by antimony in...Ch. 10 - Prob. 10.15QECh. 10 - Prob. 10.16QECh. 10 - Prob. 10.17QECh. 10 - Prob. 10.18QECh. 10 - Prob. 10.19QECh. 10 - Prob. 10.20QECh. 10 - Compare and contrast the molecular orbital and...Ch. 10 - Describe the bonding in molecular orbital terms...Ch. 10 - Prob. 10.23QECh. 10 - Prob. 10.24QECh. 10 - Prob. 10.25QECh. 10 - Prob. 10.26QECh. 10 - Prob. 10.27QECh. 10 - Prob. 10.28QECh. 10 - Prob. 10.29QECh. 10 - Prob. 10.30QECh. 10 - Prob. 10.31QECh. 10 - Prob. 10.32QECh. 10 - Prob. 10.33QECh. 10 - Prob. 10.34QECh. 10 - Prob. 10.35QECh. 10 - Prob. 10.36QECh. 10 - Prob. 10.37QECh. 10 - Prob. 10.38QECh. 10 - Prob. 10.39QECh. 10 - Use the VSEPR model to predict the bond angles...Ch. 10 - Prob. 10.41QECh. 10 - Prob. 10.42QECh. 10 - For each of the following molecules, complete the...Ch. 10 - Prob. 10.44QECh. 10 - Prob. 10.45QECh. 10 - Prob. 10.46QECh. 10 - Indicate which molecules are polar and which are...Ch. 10 - Prob. 10.48QECh. 10 - Indicate which of the following molecules are...Ch. 10 - Prob. 10.50QECh. 10 - Prob. 10.51QECh. 10 - Prob. 10.52QECh. 10 - Prob. 10.53QECh. 10 - Prob. 10.54QECh. 10 - Prob. 10.55QECh. 10 - Prob. 10.56QECh. 10 - Prob. 10.57QECh. 10 - Prob. 10.58QECh. 10 - Prob. 10.59QECh. 10 - Prob. 10.60QECh. 10 - Prob. 10.61QECh. 10 - Prob. 10.62QECh. 10 - Prob. 10.63QECh. 10 - Prob. 10.64QECh. 10 - Prob. 10.65QECh. 10 - Prob. 10.66QECh. 10 - Prob. 10.67QECh. 10 - Prob. 10.68QECh. 10 - Prob. 10.69QECh. 10 - Prob. 10.70QECh. 10 - Prob. 10.71QECh. 10 - Prob. 10.72QECh. 10 - Identify the orbitals on each of the atoms that...Ch. 10 - Prob. 10.74QECh. 10 - Prob. 10.75QECh. 10 - How many sigma bonds and how many pi bonds are...Ch. 10 - Give the hybridization of each central atom in the...Ch. 10 - Prob. 10.78QECh. 10 - Prob. 10.79QECh. 10 - Prob. 10.80QECh. 10 - Prob. 10.81QECh. 10 - Predict the hybridization at each central atom in...Ch. 10 - Prob. 10.83QECh. 10 - Tetrafluoroethylene, C2F4, is used to produce...Ch. 10 - Prob. 10.85QECh. 10 - Prob. 10.86QECh. 10 - Prob. 10.87QECh. 10 - Prob. 10.88QECh. 10 - Prob. 10.89QECh. 10 - Prob. 10.90QECh. 10 - Prob. 10.91QECh. 10 - Prob. 10.92QECh. 10 - Prob. 10.93QECh. 10 - Prob. 10.94QECh. 10 - Prob. 10.95QECh. 10 - Prob. 10.96QECh. 10 - Prob. 10.97QECh. 10 - Prob. 10.98QECh. 10 - The molecular orbital diagram of NO shown in...Ch. 10 - The molecular orbital diagram of NO shown in...Ch. 10 - The molecular orbital diagram of NO shown in...Ch. 10 - Prob. 10.102QECh. 10 - Prob. 10.103QECh. 10 - Prob. 10.104QECh. 10 - Prob. 10.105QECh. 10 - Following are the structures of three isomers of...Ch. 10 - The ions ClF2 and ClF2+ have both been observed....Ch. 10 - Aspirin, or acetylsalicylic acid, has the formula...Ch. 10 - Aspartame is a compound that is 200 times sweeter...Ch. 10 - Prob. 10.110QECh. 10 - Prob. 10.111QECh. 10 - Calcium cyanamide, CaNCN, is used both to kill...Ch. 10 - Histidine is an essential amino acid that the body...Ch. 10 - Formamide, HC(O)NH2, is prepared at high pressures...Ch. 10 - Prob. 10.115QECh. 10 - Prob. 10.116QECh. 10 - Prob. 10.117QECh. 10 - Prob. 10.118QECh. 10 - Prob. 10.119QECh. 10 - Prob. 10.120QECh. 10 - Prob. 10.121QECh. 10 - Prob. 10.122QECh. 10 - Prob. 10.123QECh. 10 - Prob. 10.124QECh. 10 - Two compounds have the formula S2F2. Disulfur...Ch. 10 - Prob. 10.126QECh. 10 - Prob. 10.127QE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- State two similarities between fluorescence and phosphorescence.arrow_forwardState three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forwardIn a photochemical reaction, how is the rate of the process related to its quantum yield?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY