
Thermodynamics, Statistical Thermodynamics, & Kinetics
3rd Edition
ISBN: 9780321766182
Author: Thomas Engel, Philip Reid
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 1, Problem 1.2CP
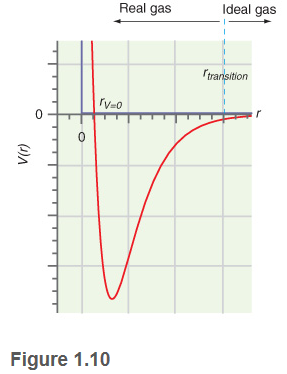
The parameter a in the van der Waals equation is greater for
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
HAND DRAW
Predict the major products of the following organic reaction:
Some important notes:
Δ
CN
?
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are
enantiomers.
ONO reaction.
Click and drag to start drawing a structure.
The following product was made from diethyl ketone and what other reagent(s)?
£
HO
10
2-pentyne
1-butyne and NaNH2
☐ 1-propanol
☐ pyridine
butanal
☐ pentanoate
Chapter 1 Solutions
Thermodynamics, Statistical Thermodynamics, & Kinetics
Ch. 1 - Real walls are never totally adiabatic. Use your...Ch. 1 - The parameter a in the van der Waals equation is...Ch. 1 - Give an example based on molecule—molecule...Ch. 1 - Prob. 1.4CPCh. 1 - Prob. 1.5CPCh. 1 - The location of the boundary between the system...Ch. 1 - Prob. 1.7CPCh. 1 - At sufficiently high temperatures, the van der...Ch. 1 - Prob. 1.9CPCh. 1 - Prob. 1.10CP
Ch. 1 - Prob. 1.11CPCh. 1 - Prob. 1.12CPCh. 1 - Prob. 1.13CPCh. 1 - The mass of a He atom is less than that of an Ar...Ch. 1 - Prob. 1.15CPCh. 1 - Prob. 1.1NPCh. 1 - A compressed cylinder of gas contains 2.74103g of...Ch. 1 - Calculate the pressure exerted by Ar for a molar...Ch. 1 - A sample of propane C3H8 is placed in a closed...Ch. 1 - A gas sample is known to be a mixture of ethane...Ch. 1 - One liter of fully oxygenated blood can carry 0.18...Ch. 1 - Yeast and other organisms can convert glucose...Ch. 1 - A vessel contains 1.15 g liq H2O in equilibrium...Ch. 1 - Consider a 31.0 L sample of moist air at 60.C and...Ch. 1 - Prob. 1.10NPCh. 1 - Prob. 1.11NPCh. 1 - A rigid vessel of volume 0.400m3 containing H2 at...Ch. 1 - A mixture of oxygen and hydrogen is analyzed by...Ch. 1 - An athlete at high performance inhales 3.75L of...Ch. 1 - Devise a temperature scale, abbreviated G, for...Ch. 1 - Aerobic cells metabolize glucose in the...Ch. 1 - Prob. 1.17NPCh. 1 - A mixture of 2.10103g of O2, 3.88103mol of N2, and...Ch. 1 - Prob. 1.19NPCh. 1 - Prob. 1.20NPCh. 1 - An initial step in the biosynthesis of glucose...Ch. 1 - Prob. 1.22NPCh. 1 - Assume that air has a mean molar mass of 28.9gmol1...Ch. 1 - When Julius Caesar expired, his last exhalation...Ch. 1 - Calculate the number of molecules per m3 in an...Ch. 1 - Prob. 1.26NPCh. 1 - A mixture of H2 and NH3 has a volume of 139.0cm3...Ch. 1 - A sealed flask with a capacity of 1.22dm3 contains...Ch. 1 - A balloon filled with 11.50 L of Ar at 18.7C and 1...Ch. 1 - Carbon monoxide competes with oxygen for binding...Ch. 1 - The total pressure of a mixture of oxygen and...Ch. 1 - Suppose that you measured the product PV of 1 mol...Ch. 1 - Liquid N2 has a density of 875.4kgm3 at its normal...Ch. 1 - Calculate the volume of all gases evolved by the...Ch. 1 - Prob. 1.35NPCh. 1 - A glass bulb of volume 0.198 L contains 0.457 g of...Ch. 1 - Prob. 1.37NPCh. 1 - Prob. 1.38NPCh. 1 - Many processes such as the fabrication of...Ch. 1 - Rewrite the van der Waals equation using the molar...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Identify me theme or themes exemplified by (a) the sharp quills of a porcupine (b) the development of a multice...
Campbell Biology in Focus (2nd Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which pair of reagents will form the given product? OH X + Y a. CH3 b. CH2CH3 ༧་་ C. CH3- CH2CH3 d.o6.(རི॰ e. CH3 OCH2CH3 -MgBr f. CH3-MgBr g. CH3CH2-MgBr -C-CH3 CH2CH3arrow_forwardQuestion 3 What best describes the product of the following reaction? 1. CH3CH2MgBr (2 eq) 2. H a new stereocenter will not be formed a new stereocenter will be formed an alkyl halide will result an alkane will result an aromatic compound will result 1 ptsarrow_forwardRank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forward
- Question 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forwardCould you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forwardWhat are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forward
- Provide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forwardPlease use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forwarda) b) c) H NaOH heat, dehydration + KOH heat, dehydration NaOH + (CH3)3CCHO heat, dehydration Pharrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
DISTINCTION BETWEEN ADSORPTION AND ABSORPTION; Author: 7activestudio;https://www.youtube.com/watch?v=vbWRuSk-BhE;License: Standard YouTube License, CC-BY
Difference Between Absorption and Adsorption - Surface Chemistry - Chemistry Class 11; Author: Ekeeda;https://www.youtube.com/watch?v=e7Ql2ZElgc0;License: Standard Youtube License