
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN: 9781305387102
Author: Kreith, Frank; Manglik, Raj M.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.16P
Water at a temperature of 77°C is to be evaporated slowly in a vessel. The water is in a low-pressure container surrounded by steam as shown in the sketch below. The steam is condensing at 107°C. The overall heat transfer coefficient between the water and the steam is
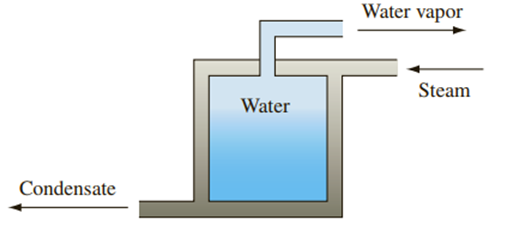
Problem 1.16
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Air enters the 1-m2 inlet of an aircraft engine at 100 kPa and 20°C with a velocity of 184 m/s. Determine the volume flow rate, in m3/s, at the engine’s inlet and the mass flow rate, in kg/s, at the engine’s exit. The gas constant of air is R = 0.287 kPa·m3/kg·K.
The volume flow rate at the engine’s inlet m3/s.
The mass flow rate at the engine’s exit is kg/s.
The ventilating fan of the bathroom of a building has a volume flow rate of 33 L/s and runs continuously. If the density of air inside is 1.20 kg/m3, determine the mass of air vented out in one day.
The mass of air is kg.
A steady-flow compressor is used to compress helium from 15 psia and 70°F at the inlet to 200 psia and 600°F at the outlet. The outlet area and velocity are 0.01 ft2 and 100 ft/s, respectively, and the inlet velocity is 53 ft/s. Determine the mass flow rate and the inlet area. The gas constant of helium is R = 2.6809 psia·ft3/lbm·R.
The mass flow rate is lbm/s.
The inlet area is ft2.
Chapter 1 Solutions
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
Ch. 1 - 1.1 On a cold winter day, the outer surface of a...Ch. 1 - 1.2 The weight of the insulation in a spacecraft...Ch. 1 - 1.3 A furnace wall is to be constructed of brick...Ch. 1 - 1.4 To measure thermal conductivity, two similar...Ch. 1 - To determine the thermal conductivity of a...Ch. 1 - A square silicon chip 7mm7mm in size and 0.5-mm...Ch. 1 - A cooling system is to be designed for a food...Ch. 1 - 1.80 Describe and compare the modes of heat loss...Ch. 1 - Heat is transferred at a rate of 0.1 kW through...Ch. 1 - 1.10 A heat flux meter at the outer (cold) wall of...
Ch. 1 - 1.11 Calculate the heat loss through a glass...Ch. 1 - 1.12 A wall with a thickness is made of a...Ch. 1 - 1.13 If the outer air temperature in Problem is...Ch. 1 - Using Table 1.4 as a guide, prepare a similar...Ch. 1 - 1.15 A thermocouple (0.8-mm-diameter wire) used to...Ch. 1 - Water at a temperature of 77C is to be evaporated...Ch. 1 - The heat transfer rate from hot air by convection...Ch. 1 - The heat transfer coefficient for a gas flowing...Ch. 1 - 1.19 A cryogenic fluid is stored in a...Ch. 1 - A high-speed computer is located in a...Ch. 1 - 1.21 In an experimental set up in a laboratory, a...Ch. 1 - 1.22 In order to prevent frostbite to skiers on...Ch. 1 - Using the information in Problem 1.22, estimate...Ch. 1 - Two large parallel plates with surface conditions...Ch. 1 - 1.25 A spherical vessel, 0.3 m in diameter, is...Ch. 1 - 1.26 Repeat Problem 1.25 but assume that the...Ch. 1 - Determine the rate of radiant heat emission in...Ch. 1 - 1.28 The sun has a radius of and approximates a...Ch. 1 - 1.29 A spherical interplanetary probe with a 30-cm...Ch. 1 - A spherical communications satellite, 2 m in...Ch. 1 - A long wire 0.7 mm in diameter with an emissivity...Ch. 1 - Wearing layers of clothing in cold weather is...Ch. 1 - A section of a composite wall with the dimensions...Ch. 1 - A section of a composite wall with the dimensions...Ch. 1 - Repeat Problem 1.35 but assume that instead of...Ch. 1 - 1.37 Mild steel nails were driven through a solid...Ch. 1 - Prob. 1.38PCh. 1 - 1.39 On a cold winter day, the outside wall of a...Ch. 1 - As a designer working for a major electric...Ch. 1 - 1.41 A heat exchanger wall consists of a copper...Ch. 1 - 1.43 A simple solar heater consists of a flat...Ch. 1 - A composite refrigerator wall is composed of 5 cm...Ch. 1 - An electronic device that internally generates 600...Ch. 1 - 1.47 A flat roof is modeled as a flat plate...Ch. 1 - A horizontal, 3-mm-thick flat-copper plate, 1-m...Ch. 1 - 1.49 A small oven with a surface area of is...Ch. 1 - A steam pipe 200 mm in diameter passes through a...Ch. 1 - 1.51 The inner wall of a rocket motor combustion...Ch. 1 - 1.52 A flat roof of a house absorbs a solar...Ch. 1 - Determine the power requirement of a soldering...Ch. 1 - 1.54 The soldering iron tip in Problem 1.53...Ch. 1 - Prob. 1.55PCh. 1 - A pipe carrying superheated steam in a basement at...Ch. 1 - Draw the thermal circuit for heat transfer through...Ch. 1 - 1.60 Two electric resistance heaters with a 20 cm...Ch. 1 - 1.63 Liquid oxygen (LOX) for the space shuttle is...Ch. 1 - The interior wall of a large, commercial walk-in...Ch. 1 - 1.67 In beauty salons and in homes, a ubiquitous...Ch. 1 - The heat transfer coefficient between a surface...Ch. 1 - The thermal conductivity of fibreglass insulation...Ch. 1 - 1.71 The thermal conductivity of silver at 212°F...Ch. 1 - 1.72 An ice chest (see sketch) is to constructed...Ch. 1 - Estimate the R-values for a 5-cm-thick fiberglass...Ch. 1 - A manufacturer in the United States wants to sell...Ch. 1 - Referring to Problem 1.74, how many kilograms of...Ch. 1 - 1.76 Explain a fundamental characteristic that...Ch. 1 - 1.77 Explain each in your own words. (a) What is...Ch. 1 - What are the important modes of heat transfer for...Ch. 1 - 1.79 Consider the cooling of (a) a personal...Ch. 1 - Describe and compare the modes of heat loss...Ch. 1 - A person wearing a heavy parka is standing in a...Ch. 1 - Discuss the modes of heat transfer that determine...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 1. The maximum and minimum stresses as well as the shear stress seen subjected the piece in plane A-A. Assume it is a cylinder with a diameter of 12.7mm 2. Draw the Mohr circle for the stress state using software. 3. Selection of the material for the prosthesis, which must be analyzed from the point of safety and cost view.arrow_forwardMarrow_forward× Your answer is incorrect. (Manometer) Determine the angle 0 of the inclined tube shown in figure below if the pressure at A is 1 psi greater than that at B. 1ft SG=0.61 十 A Ꮎ 1ft SG=1.0 8.8 ft 0 = Hi 15.20 deg Airarrow_forward
- I don't know how to solve thisarrow_forward1. The maximum and minimum stresses as well as the shear stress seen subjected the piece in plane A-A. Assume it is a cylinder with a diameter of 12.7mm 2. Draw the Mohr circle for the stress state using software. 3. Selection of the material for the prosthesis, which must be analyzed from the point of safety and cost view.arrow_forwardFirst, define the coordinate system XY with its origin at O2 and X-axis passing through O4 asshown above, then based on the provided steps Perform coordinate transformation from XY to xy to get the trajectory of point P. Show all the steps and calcualtionsarrow_forward
- I don't know how to solve thisarrow_forwardQuestion 2 (40 Points) Consider the following double pendulum-like system with links ₁ and 12. The angles 0 and & could have angular velocities ėêk and êk, respectively, where ②k is a unit vector that points out of the page and is perpendicular to x and y. They could also have angular accelerations Ök and êk. The angle is defined relative to the angle 0. The link 12 is a spring and can extend or compress at a rate of 12. It can also have a rate of extension or compression Ï2. li y êr1 êe 12 χ 3 еф er2 ده لج 1) Express the velocity of the mass in terms of the unit vectors ê0, êr1, êø, and êr2, and any extension/contraction of the links (e.g.,. i; and Ï¿) (12 Points) 2) Express the acceleration of the mass in terms of the unit vectors ê¤, ê×1, êp, and êÃ2, and any extension/contraction of the links (e.g.,. İ; and Ï¿) (12 Points) 3) Express the velocity of the mass in terms of unit vectors î and ĵ that point in the x and y directions, respectively. Also include the appropriate,…arrow_forwardprovide step by step solutions for angles teta 3 and teta 4 by the vector loopmethod. Show work in: vector loop, vector equations, solution procedure.arrow_forward
- (Manometer) A tank is constructed of a series of cylinders having diameters of 0.35, 0.30, and 0.20 m as shown in the figure below. The tank contains oil, water, and glycerin and a mercury manometer is attached to the bottom as illustrated. Calculate the manometer reading, h. 0.11 m + SAE 30 Oil 0.13 m + Water 0.10 m Glycerin + 0.10 m Mercury h = marrow_forwardP = A piston having a cross-sectional area of 0.40 m² is located in a cylinder containing water as shown in the figure below. An open U-tube manometer is connected to the cylinder as shown. For h₁ = 83 mm and h = 111 mm what is the value of the applied force, P, acting on the piston? The weight of the piston is negligible. Hi 5597.97 N P Piston Water Mercuryarrow_forwardStudent Name: Student Id: College of Applied Engineering Al-Muzahmiyah Branch Statics (AGE 1330) Section-1483 Quiz-2 Time: 20 minutes Date: 16/02/2025 Q.1. A swinging door that weighs w=400.0N is supported by hinges A and B so that the door can swing about a vertical' axis passing through the hinges (as shown in below figure). The door has a width of b=1.00m and the door slab has a uniform mass density. The hinges are placed symmetrically at the door's edge in such a way that the door's weight is evenly distributed between them. The hinges are separated by distance a=2.00m. Find the forces on the hinges when the door rests half-open. Draw Free body diagram also. [5 marks] [CLO 1.2] Mool b ర a 2.0 m B 1.0 marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning

Principles of Heat Transfer (Activate Learning wi...
Mechanical Engineering
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Cengage Learning
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=NyOYW07-L5g;License: Standard youtube license