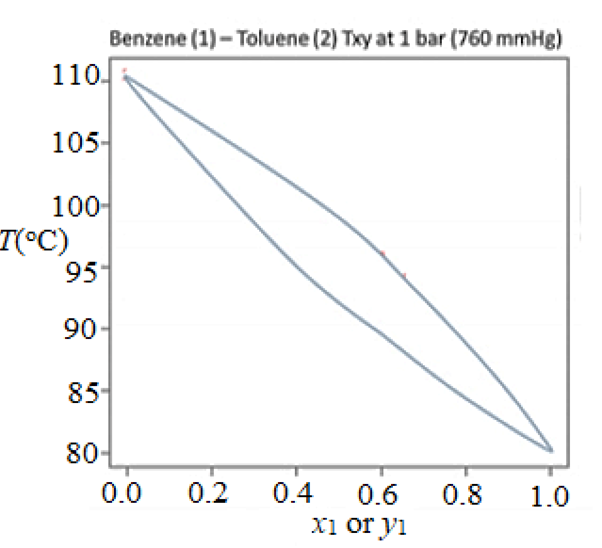
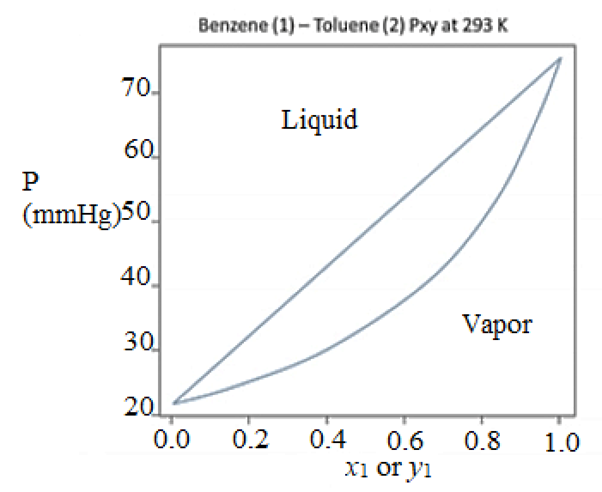
3. Use the Txy and Pxy phase diagrams for benzene-toluene to answer the questions that follow. Benzene (1)- Toluene (2) Txy at 1 bar (760 mmHg) Benzene (1) – Toluene (2) Pxy at 293 K (mmHg) 04 06 02 00 02 08 10 00 04 06 08 X, or y. X1 or y1 a. What is the phase(s) of an overall 20% toluene, 80% benzene mixture at 293 K and 30 mmHg, and if two phases are present, what is their composition? What is the highest temperature at which a 60% toluene, 40% benzene vapor would begin to condense at 1 bar (760 mmHg)? b. An 80% benzene, 20% toluene liquid mixture is heated at 1 bar (760 mmHg) in a closed piston chamber. What will be the temperature when the last drop of liquid is vaporized? c. A piston operating at a constant temperature of 293 K is initially filled with a liquid containing 80 moles of benzene and 120 moles of toluene. The volume of the piston is expanded until the pressure inside the piston is 35 mmHg. What will be the phase(s) of the mixture, and if two phases are present, how many moles of each species are in each phase? 06 08
3. Use the Txy and Pxy phase diagrams for benzene-toluene to answer the questions that follow. Benzene (1)- Toluene (2) Txy at 1 bar (760 mmHg) Benzene (1) – Toluene (2) Pxy at 293 K (mmHg) 04 06 02 00 02 08 10 00 04 06 08 X, or y. X1 or y1 a. What is the phase(s) of an overall 20% toluene, 80% benzene mixture at 293 K and 30 mmHg, and if two phases are present, what is their composition? What is the highest temperature at which a 60% toluene, 40% benzene vapor would begin to condense at 1 bar (760 mmHg)? b. An 80% benzene, 20% toluene liquid mixture is heated at 1 bar (760 mmHg) in a closed piston chamber. What will be the temperature when the last drop of liquid is vaporized? c. A piston operating at a constant temperature of 293 K is initially filled with a liquid containing 80 moles of benzene and 120 moles of toluene. The volume of the piston is expanded until the pressure inside the piston is 35 mmHg. What will be the phase(s) of the mixture, and if two phases are present, how many moles of each species are in each phase? 06 08
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question

Transcribed Image Text:3. Use the Txy and Pxy phase diagrams for benzene-toluene to answer the questions that follow.
Benzene (1) – Toluene (2) Txy at 1 bar (760 mmHg)
Benzene (1) – Toluene (2) Pxy at 293 K
(mmHg)
00
0.2
04
0.6
0.8
1.0
00
02
04
06
0.8
1.0
X, or y,
X1 or y1
a. What is the phase(s) of an overall 20% toluene, 80% benzene mixture at 293 K and 30
mmHg, and if two phases are present, what is their composition? What is the highest
temperature at which a 60% toluene, 40% benzene vapor would begin to condense at 1 bar
(760 mmHg)?
b. An 80% benzene, 20% toluene liquid mixture is heated at 1 bar (760 mmHg) in a closed
piston chamber. What will be the temperature when the last drop of liquid is vaporized?
c. A piston operating at a constant temperature of 293 K is initially filled with a liquid
containing 80 moles of benzene and 120 moles of toluene. The volume of the piston is
expanded until the pressure inside the piston is 35 mmHg. What will be the phase(s) of the
mixture, and if two phases are present, how many moles of each species are in each phase?
(ɔ.) I
105 110
Expert Solution
Step 1
The graphs are given for the benzene (1) – toluene (2) system at 1 bar and 293 K are:
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The