Q3. The following information on the pressure-temperature diagram of nitrogen: P (atm) T(K) 0.123 63.15 33.3978 126.19 1.0 77.35 Triple point Critical point Normal boiling point Density of N₂(s): 1.03g/cm-³ N₂(1): 0.808g/cm-³ Using this information, sketch a phase-equilibrium diagram of nitrogen and mark each point. Then, describe why the slope of each phase/phase equilibrium is positive or negative.
Q3. The following information on the pressure-temperature diagram of nitrogen: P (atm) T(K) 0.123 63.15 33.3978 126.19 1.0 77.35 Triple point Critical point Normal boiling point Density of N₂(s): 1.03g/cm-³ N₂(1): 0.808g/cm-³ Using this information, sketch a phase-equilibrium diagram of nitrogen and mark each point. Then, describe why the slope of each phase/phase equilibrium is positive or negative.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question

Transcribed Image Text:Q3. The following information on the pressure-temperature diagram of nitrogen:
P (atm)
T(K)
0.123
33.3978
1.0
Triple point
Critical point
Normal boiling point
Density of N₂(s): 1.03g/cm-³
N₂(1): 0.808g/cm-³
63.15
126.19
77.35
Using this information, sketch a phase-equilibrium diagram of nitrogen and mark each point. Then,
describe why the slope of each phase/phase equilibrium is positive or negative.
Expert Solution
Step 1
The phase diagram indicating the above points can be created as:
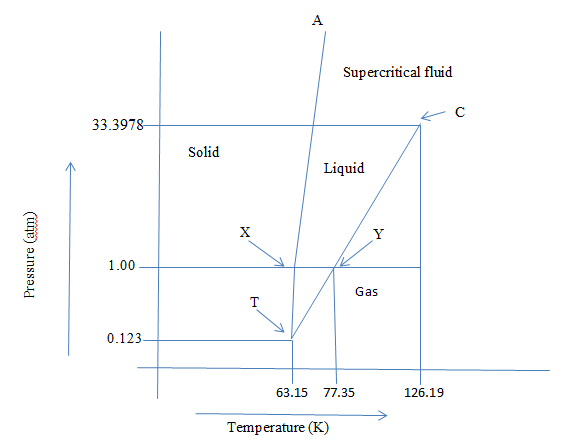
X is the normal melting point, Y is the normal boiling point, T is a triple point and C is the critical point.
The density of liquid nitrogen is less than that of solid, so the solid-liquid line named TA is tilted towards the liquid which indicates that it expands on heating.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The