
(a)
Interpretation :
Reactant and product graphs must be labeled.
Concept Introduction :
Concentration of reactants gradually decreases and that of product increases.
(a)
Answer to Problem 5E
Blue and green graphs are for reactants. Red graph is for product.
Explanation of Solution
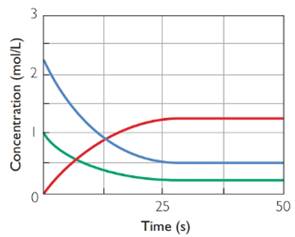
As per the graphs it is clear that with time blue and green graphs are coming downward, i.e., concentration decreases. Red graph is going upward, i.e., concentration is increasing. Thus blue and green graphs are for reactants and red graph is for product.
b)
Interpretation :
Equilibrium concentrations of reactant and product must be read from the graph.
Concept Introduction :
At equilibrium
b)
Answer to Problem 5E
Equilibrium concentrations of blue graph and green graph reactants are 0.5 M and 0.25 M respectively. equilibrium concentration of product (red graph) is 1.25 M.
Explanation of Solution
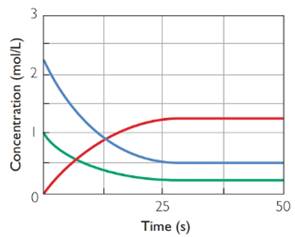
Equilibrium concentration is the concentration where the graphs are parallel to x-axis(time). At this point there is no change in concentration as the forward and backward reactions are taking place in same rate. From the graphs all the concentrations are found.
c)
Interpretation :
The time taken to reach equilibrium must be found out.
Concept Introduction :
Time when the forward reaction and backward reaction is taking place in same speed then equilibrium is attained.
c)
Answer to Problem 5E
It took around 25 s to reach equilibrium.
Explanation of Solution
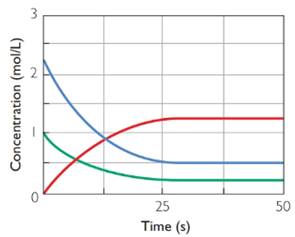
From the graphs it is clear that all the three graphs become parallel after 25 s. At this time point forward and backward reaction is taking place in same speed. So it took 25 s for the attainment of equilibrium.
d)
Interpretation :
The variable which is equal at equilibrium must be explained.
Concept Introduction :
At equilibrium rate of forward and backward reactions are same.
d)
Answer to Problem 5E
At equilibrium rate of forward reaction is “equal” to rate of backward reaction.
Explanation of Solution
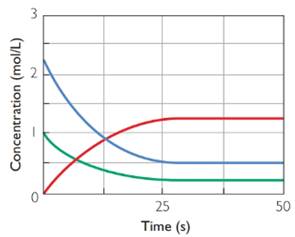
After 25 s the reaction has attained equilibrium. At this point all the three graphs are parallel to time axis which means there is no change in concentration of reactants or product with time. So rate of forward reaction is “equal” to rate of backward reaction at equilibrium.
Chapter U6 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Biology: Life on Earth (11th Edition)
Human Physiology: An Integrated Approach (8th Edition)
Campbell Biology in Focus (2nd Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Microbiology: An Introduction
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- Question 5: Name the following compound in two ways using side chain and using prefix amine (Common name and IUPAC name both) CH3NH2 CH3CH2NHCH3 CH₂CH₂N(CH3)2 Draw the structure of diethyl methyl amine Question 6. Write the balanced combustion reaction for: a. Hexane b. Propyne c. 2-pentene Question 7: Write the following electrophilic substitution reactions of benzene: Hint: Use notes if you get confused a. Halogenation reaction: b. Nitration reaction : c. Sulphonation reaction: d. Alkylation reaction: e. Aceylation reaction:arrow_forwardQuestion 4. Name the following structures ○ CH3-C-N-H H CH3CH2-C-N-H H CH3CH2-C-N-CH3 Harrow_forwardA. Add Water to below compound which 2-methyl 2-butene (addition Reaction) H₂C CH₂ CH, + H₂O-> ? Major product? Minor product? B. Add Bromine to the compound which 2-methyl 2-butene (addition Reaction) CH₂ CH₂ + Br₂→ ? Major product and Minor product both are same in this? C. Add Hydrogen Bromide to the compound which 2-methyl 2-butene (addition Reaction) H,C CH₂ CH₂ + HBr Major product? Minor product? D. Add Hydrogen to the compound which 2-methyl 2-butene (addition Reaction) CH₂ CH₂ + H₂ Major product and Minor product both are same in this?arrow_forward
- 36) Complete the following multi-step reactions showing applications of enolate ions arising from ketones, esters, malonic ester, and keto ester, etc. (30 pts) (1) A NaOH, H₂O+ heat A NaOEt EtO OEt (11) EOH, H+ H. B LDA, H₂O+ -78°C B (i) NaOMe, Et-Br (ii) H₂O+, heat EtOOC (III) COOEt B A (i) NaOEt LiAlH 4-bromo-2-butene H₂O+ (ii) H3O+, heat Write the mechanism for Aldol Condensation (I A or B), and Claisen Condensation (II A).arrow_forward31) Complete two sets of reactions involving (R)-4-methyl-pent-2-ol producing racemic mixture of tertiary alcohols (D) and ketone derivative (C). Illustrate the mechanism of B and C or D. (25 pts) O OH 0 K2Cr2O7 Ph-CH2-Br, Mg, H2SO4 THF, H3O* (A) (D) Racemic mixture TsCl, Py (B) KCN, DMSO Ph-CH2-Br, Mg, THF, H3O+ (C) Mechanism for reactions B and C:arrow_forwardManoharan Mariappan, Ph.D., Dept. of Natur. Sci., NFC, Tallahassee, FL 33) Synthesize the aromatic compound containing para-substituted carbonyl compound starting from benzene. Illustrate the mechanism for reaction A. 1) NU (25 pts) A FeCl B (i) HNO3, H2SO4 (II) Sn, HCl(aq) NH₂ NO₂-D NH₂ (i) MeCO2Me, heat C (ii) K2Cr2O7/H2SO4 D (ii) SOCl2 (iii) 2 Et-NH2 Mechanism for reaction for the nitration of alkyl benzene (B-i): Characterize the product compound arising from the reaction D by IR and IH NMR spectral data: IR data (cm): 'H NMR data: Draw the structure and assign the chemical shift with the spin-splitting.arrow_forward
- Write structural formulas for the major products by doing addition reactions 1. You must add H2 as Pt is catalyst it does not take part in reactions only speed up the process H₂ CH2=CH-CH3 Pt 2. Add HCI break it into H and Cl CH3 HCI 3. Add Br2 only CC14 is catalyst CH3-CH=CH2 B12 CCl4 4. Add water to this and draw major product, H2SO4 is catalyst you have add water H20 in both the reaction below H₂SO4 CH3-CH=CH2 CH3 H2SO4/H₂O CH3-C=CH2 reflux ?arrow_forwardPlan the synthesis of the following compound using the starting material provided and any other reagents needed as long as carbon based reagents have 3 carbons or less. Either the retrosynthesis or the forward synthesis (mechanisms are not required but will be graded if provided) will be accepted if all necessary reagents and intermediates are shown (solvents and temperature requirements are not needed unless specifically involved in the reaction, i.e. DMSO in the Swern oxidation or heat in the KMnO4 oxidation). H Harrow_forwardHint These are benzene substitution reactions. ALCI3 and UV light are catalyst no part in reactions and triangle A means heating. A. Add ethyl for Et in benzene ring alkylation reaction EtCl = CH3CH2CL 1) EtC1 / AlCl3 / A ? B: Add Br to benzene ring ( substitution) 2) Br₂ / uv light ? C Add (CH3)2 CHCH2 in benzene ring ( substitution) (CH3)2CHCH,C1 / AICI, ?arrow_forward
- Draw the mechanism to make the alcohol 2-hexanol. Draw the Mechanism to make the alcohol 1-hexanol.arrow_forwardDraw the mechanism for the formation of diol by starting with 1-pentanal in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forwardIdentify each chiral carbon as either R or S. Identify the overall carbohydrates as L or Darrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





