
The Greenhouse Effect and Global Warming
Although seasons come and go, on average the earth’s climate is very steady. To maintain this stability, the earth must radiate thermal energy—electromagnetic waves—back into space at exactly the same average rate that it receives energy from the sun. Because the earth is much cooler than the sun, its thermal radiation is long-wavelength infrared radiation that we cannot see. A straightforward calculation using Stefan's law finds that the average temperature of the earth should be –18°C, or 0°F, for the incoming and outgoing radiation to lie in balance.
This result is clearly not correct; at this temperature, the entire earth would be covered in snow and ice. The measured global average temperature is actually a balmier 15°C, or 59°F. The straightforward calculation fails because it neglects to consider the earth’s atmosphere. At visible wavelengths, as the figure shows, the atmosphere has a wide “window” of transparency, but this is not true at the infrared wavelengths of the earth’s thermal radiation. The atmosphere lets in the visible radiation from the sun, but the outgoing thermal radiation from the earth sees a much smaller “window.” Most of this radiation is absorbed in the atmosphere.
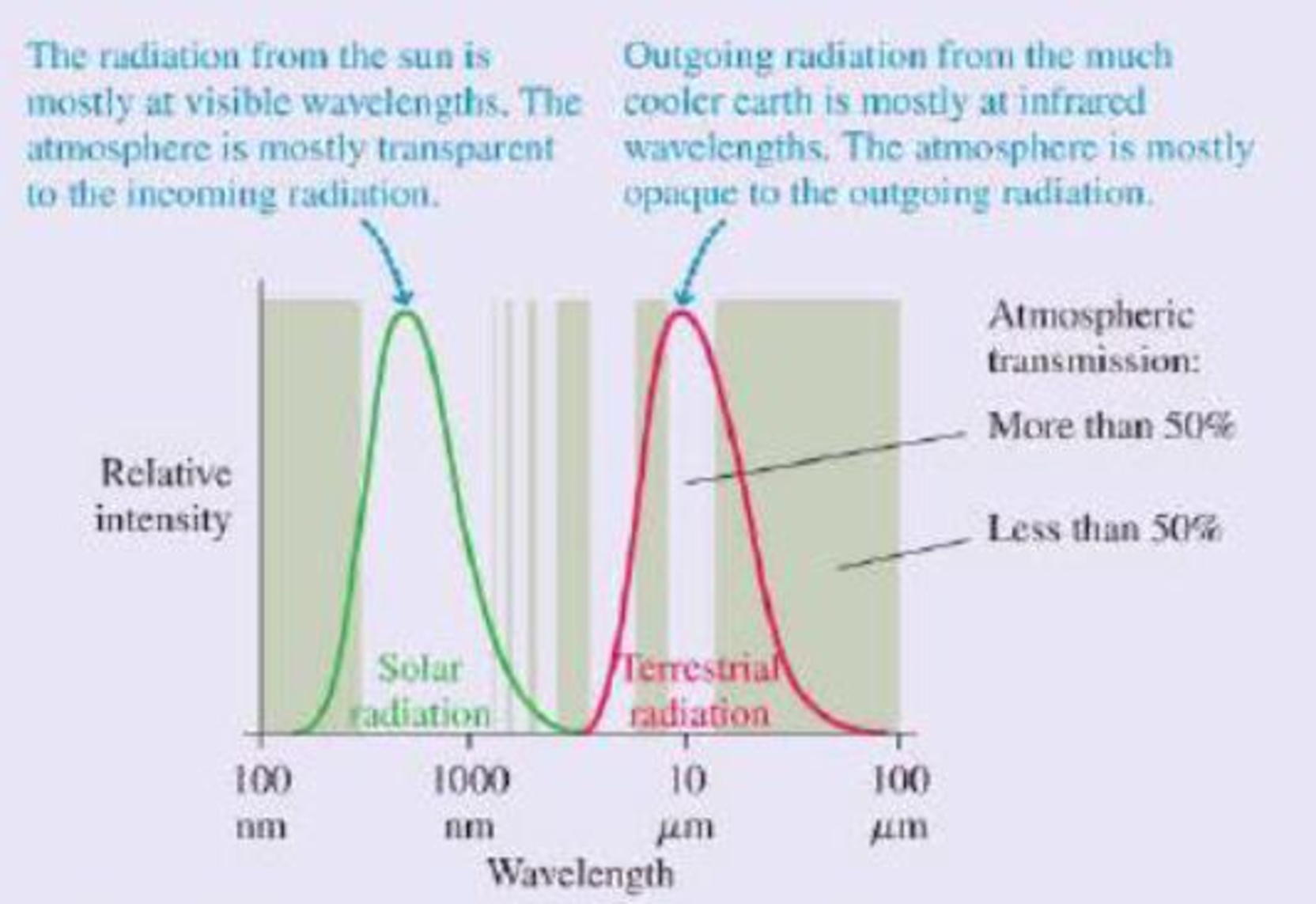
Thermal radiation curves for the sun and the earth. The shaded bands show regions for which the atmosphere is transparent (no shading) or opaque (shaded) to electromagnetic radiation.
Because it’s easier for visible radiant energy to get in than for infrared to get out, the earth is warmer than it would be without the atmosphere. The additional warming of the earth’s surface because of the atmosphere is called the greenhouse effect. The greenhouse effect is a natural part of the earth’s physics; it has nothing to do with human activities, although it’s doubtful any advanced life forms would have evolved without it.
The atmospheric gases most responsible for the greenhouse effect are carbon dioxide and water vapor, both strong absorbers of infrared radiation. These greenhouse gases are of concern today because humans, through the burning of fossil fuels (oil, coal, and natural gas), are rapidly increasing the amount of carbon dioxide in the atmosphere. Preserved air samples show that carbon dioxide made up 0.027% of the atmosphere before the industrial revolution. In the last 150 years, human activities have increased the amount of carbon dioxide by nearly 50%, to about 0.040%. By 2050, the carbon dioxide concentration will likely increase to 0.054%, double the pre-industrial value, unless the use of fossil fuels is substantially reduced.
Carbon dioxide is a powerful absorber of infrared radiation. And good absorbers are also good emitters. The carbon dioxide in the atmosphere radiates energy back to the surface of the earth, warming it. Increasing the concentration of carbon dioxide in the atmosphere means more radiation: this increases the average surface temperature of the earth. The net result is global warming.
There is strong evidence that (he earth has warmed nearly 1°C in the last 100 years because of increased greenhouse gases. What happens next? Climate scientists, using sophisticated models of the earth’s atmosphere and oceans, calculate that a doubling of the carbon dioxide concentration will likely increase the earth’s average temperature by an additional 2°C (≈ 3°F) to 6°C (≈9°F) There is some uncertainty in these calculations; the earth is a large and complex system. Perhaps the earth will get cloudier as the temperature increases, moderating the increase. Or perhaps the arctic ice cap will melt, making the earth less reflective and leading to an even more dramatic
But the basic physics that leads to the greenhouse effect, and to global warming, is quite straightforward. Carbon dioxide in the atmosphere keeps the earth warm; more carbon dioxide will make it warmer. How much warmer? That’s an important question, one that many scientists around the world are attempting to answer with ongoing research. But large or small, change is coming. Global warming is one of the most serious challenges facing scientists, engineers, and all citizens in the 21st century.
The following questions are related to the passage “The Greenhouse Effect and Global Warming” on the previous page.
Electromagnetic waves in certain wavelength ranges interact with water molecules because the molecules have a large electric dipole moment. The electric field of the wave
- A. Exerts a net force on the water molecules.
- B. Exerts a net torque on the water molecules.
- C. Exerts a net force and a net torque on the water molecules.
Want to see the full answer?
Check out a sample textbook solution
Chapter P Solutions
Mastering Physics with Pearson eText -- Standalone Access Card -- for College Physics: A Strategic Approach (3rd Edition)
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Introductory Chemistry (6th Edition)
Chemistry (7th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Campbell Biology in Focus (2nd Edition)
Applications and Investigations in Earth Science (9th Edition)
- In the figure Q = 5.7 nC and all other quantities are accurate to 2 significant figures. What is the magnitude of the force on the charge Q? (k = 1/4πε 0 = 8.99 × 109 N · m2/C2)arrow_forwardNow add a fourth charged particle, particle 3, with positive charge q3, fixed in the yz-plane at (0,d2,d2). What is the net force F→ on particle 0 due solely to this charge? Express your answer (a vector) using k, q0, q3, d2, i^, j^, and k^. Include only the force caused by particle 3.arrow_forwardFor a tornadoes and hurricanes, which of the following is most critical? an alert a watch a warning a predictionarrow_forward
- When a warm front advances up and over a cold front, what is it called? front inversion stationary front cold front occlusion warm front occlusionarrow_forward1) Consider two positively charged particles, one of charge q0 (particle 0) fixed at the origin, and another of charge q1 (particle 1) fixed on the y-axis at (0,d1,0). What is the net force F→ on particle 0 due to particle 1? Express your answer (a vector) using any or all of k, q0, q1, d1, i^, j^, and k^. 2) Now add a third, negatively charged, particle, whose charge is −q2− (particle 2). Particle 2 fixed on the y-axis at position (0,d2,0). What is the new net force on particle 0, from particle 1 and particle 2? Express your answer (a vector) using any or all of k, q0, q1, q2, d1, d2, i^, j^, and k^. 3) Particle 0 experiences a repulsion from particle 1 and an attraction toward particle 2. For certain values of d1 and d2, the repulsion and attraction should balance each other, resulting in no net force. For what ratio d1/d2 is there no net force on particle 0? Express your answer in terms of any or all of the following variables: k, q0, q1, q2.arrow_forwardA 85 turn, 10.0 cm diameter coil rotates at an angular velocity of 8.00 rad/s in a 1.35 T field, starting with the normal of the plane of the coil perpendicular to the field. Assume that the positive max emf is reached first. (a) What (in V) is the peak emf? 7.17 V (b) At what time (in s) is the peak emf first reached? 0.196 S (c) At what time (in s) is the emf first at its most negative? 0.589 x s (d) What is the period (in s) of the AC voltage output? 0.785 Sarrow_forward
- A bobsled starts at the top of a track as human runners sprint from rest and then jump into the sled. Assume they reach 40 km/h from rest after covering a distance of 50 m over flat ice. a. How much work do they do on themselves and the sled which they are pushing given the fact that there are two men of combined mass 185 kg and the sled with a mass of 200 kg? (If you haven't seen bobsledding, watch youtube to understand better what's going on.) b. After this start, the team races down the track and descends vertically by 200 m. At the finish line the sled crosses with a speed of 55 m/s. How much energy was lost to drag and friction along the way down after the men were in the sled?arrow_forwardFor what type of force is it not possible to define a potential energy expression?arrow_forward10. Imagine you have a system in which you have 54 grams of ice. You can melt this ice and then vaporize it all at 0 C. The melting and vaporization are done reversibly into a balloon held at a pressure of 0.250 bar. Here are some facts about water you may wish to know. The density of liquid water at 0 C is 1 g/cm³. The density of ice at 0 C is 0.917 g/cm³. The enthalpy of vaporization of liquid water is 2.496 kJ/gram and the enthalpy of fusion of solid water is 333.55 J/gram.arrow_forward
- Consider 1 mole of supercooled water at -10°C. Calculate the entropy change of the water when the supercooled water freezes at -10°C and 1 atm. Useful data: Cp (ice) = 38 J mol-1 K-1 Cp (water) 75J mol −1 K -1 Afus H (0°C) 6026 J mol −1 Assume Cp (ice) and Cp (water) to be independent of temperature.arrow_forwardThe molar enthalpy of vaporization of benzene at its normal boiling point (80.09°C) is 30.72 kJ/mol. Assuming that AvapH and AvapS stay constant at their values at 80.09°C, calculate the value of AvapG at 75.0°C, 80.09°C, and 85.0°C. Hint: Remember that the liquid and vapor phases will be in equilibrium at the normal boiling point.arrow_forward3. The entropy of an ideal gas is S = Nkg In V. Entropy is a state function rather than a path function, and in this problem, you will show an example of the entropy change for an ideal gas being the same when you go between the same two states by two different pathways. A. Express ASV = S2 (V2) - S₁(V1), the change in entropy upon changing the volume from V₁to V2, at fixed particle number N and energy, U. B. Express ASN = S₂(N₂) - S₁ (N₁), the change in entropy upon changing the particle number from N₁ to N2, at fixed volume V and energy U. C. Write an expression for the entropy change, AS, for a two-step process (V₁, N₁) → (V2, N₁) → (V2, N₂) in which the volume changes first at fixed particle number, then the particle number changes at fixed volume. Again, assume energy is constant.arrow_forward
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill AstronomyPhysicsISBN:9781938168284Author:Andrew Fraknoi; David Morrison; Sidney C. WolffPublisher:OpenStax
AstronomyPhysicsISBN:9781938168284Author:Andrew Fraknoi; David Morrison; Sidney C. WolffPublisher:OpenStax





