
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter L4, Problem 5E
For each structure below, use numbers to indicate chemically equivalent and distinct hydrogens,and make a table showing the predicted integration and multiplicity of each peak cluster.
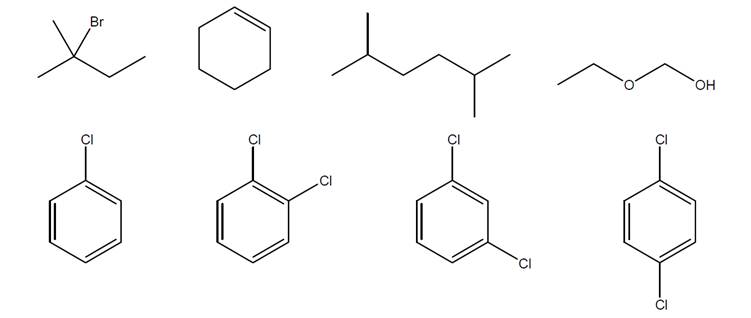
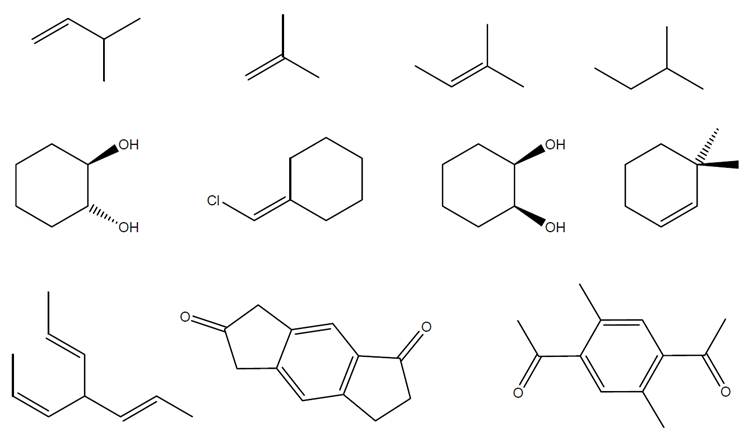
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Solve this
く
Predicting the pr
Predict the major products of the following organic reaction:
Δ
Some important notes:
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
• Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are
enantiomers.
?
Click and drag to start drawing a structure.
propose synthesis
Chapter L4 Solutions
Organic Chemistry: A Guided Inquiry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explanation O Conjugated Pi Systems Deducing the reactants of a Diels-Alder reaction Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Click and drag to start drawing a structure. Xarrow_forwardDiels Alder Cycloaddition: Focus on regiochemistry (problems E-F) –> match + of thedienophile and - of the diene while also considering stereochemistry (endo).arrow_forwardHELP! URGENT! PLEASE RESOND ASAP!arrow_forward
- Question 4 Determine the rate order and rate constant for sucrose hydrolysis. Time (hours) [C6H12O6] 0 0.501 0.500 0.451 1.00 0.404 1.50 0.363 3.00 0.267 First-order, k = 0.210 hour 1 First-order, k = 0.0912 hour 1 O Second-order, k = 0.590 M1 hour 1 O Zero-order, k = 0.0770 M/hour O Zero-order, k = 0.4896 M/hour O Second-order, k = 1.93 M-1-hour 1 10 ptsarrow_forwardDetermine the rate order and rate constant for sucrose hydrolysis. Time (hours) [C6H12O6] 0 0.501 0.500 0.451 1.00 0.404 1.50 0.363 3.00 0.267arrow_forwardDraw the products of the reaction shown below. Use wedge and dash bonds to indicate stereochemistry. Ignore inorganic byproducts. OSO4 (cat) (CH3)3COOH Select to Draw ઘarrow_forward
- Calculate the reaction rate for selenious acid, H2SeO3, if 0.1150 M I-1 decreases to 0.0770 M in 12.0 minutes. H2SeO3(aq) + 6I-1(aq) + 4H+1(aq) ⟶ Se(s) + 2I3-1(aq) + 3H2O(l)arrow_forwardProblem 5-31 Which of the following objects are chiral? (a) A basketball (d) A golf club (b) A fork (c) A wine glass (e) A spiral staircase (f) A snowflake Problem 5-32 Which of the following compounds are chiral? Draw them, and label the chirality centers. (a) 2,4-Dimethylheptane (b) 5-Ethyl-3,3-dimethylheptane (c) cis-1,4-Dichlorocyclohexane Problem 5-33 Draw chiral molecules that meet the following descriptions: (a) A chloroalkane, C5H11Cl (c) An alkene, C6H12 (b) An alcohol, C6H140 (d) An alkane, C8H18 Problem 5-36 Erythronolide B is the biological precursor of erythromycin, a broad-spectrum antibiotic. How H3C CH3 many chirality centers does erythronolide B have? OH Identify them. H3C -CH3 OH Erythronolide B H3C. H3C. OH OH CH3arrow_forwardPLEASE HELP! URGENT! PLEASE RESPOND!arrow_forward
- 2. Propose a mechanism for this reaction. ہلی سے ملی N H (excess)arrow_forwardSteps and explanationn please.arrow_forwardProblem 5-48 Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C). OH H OH HO CH2OH Ascorbic acid O H Problem 5-49 Assign R or S stereochemistry to the chirality centers in the following Newman projections: H Cl H CH3 H3C. OH H3C (a) H H H3C (b) CH3 H Problem 5-52 Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each: OH OH (a) CH3CHCH2CH2CHCH3 CH3 H3C. -OH (c) H3C CH3 (b) Problem 5-66 Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States. H2N H IHH S Cephalexin N. CH3 CO₂Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY