
Concept explainers
Compound X
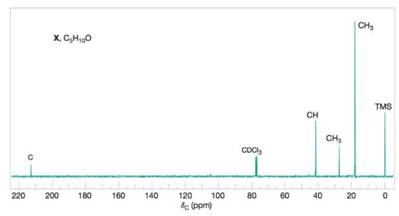
Figure 2 The broadband proton-decoupled
Want to see the full answer?
Check out a sample textbook solution
Chapter FRP Solutions
Organic Chemistry
Additional Science Textbook Solutions
Anatomy & Physiology (6th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Microbiology: An Introduction
Chemistry: Structure and Properties (2nd Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Microbiology: Principles and Explorations
- n Feb 3 A T + 4. (2 pts) Draw the structure of the major component of the Limonene isolated. Explain how you confirmed the structure. 5. (2 pts) Draw the fragment corresponding to the base peak in the Mass spectrum of Limonene. 6. (1 pts) Predict the 1H NMR spectral data of R-Limonene. Proton NMR: 5.3 pon multiplet (H Ringarrow_forwardPart VI. Ca H 10 O is the molecular formula of compound Tom and gives the in the table below. Give a possible structure for compound Tom. 13C Signals summarized C1 C2 C3 C4 C5 C6 C7 13C shift (ppm) 23.5 27.0 33.0 35.8 127 162 205 DEPT-90 + DEPT-135 + +arrow_forward2. Using the following data to calculate the value of AvapH o of water at 298K. AvapH o of water at 373K is 40.7 kJ/mol; molar heat capacity of liquid water at constant pressure is 75.2J mol-1 K-1 and molar heat capacity of water vapor at constant pressure is 33.6 J mol-1 K-1.arrow_forward
- Part VII. Below are the 'HNMR 13 3 C-NMR, COSY 2D- NMR, and HSQC 20-NMR (Similar with HETCOR but axes are reversed) spectra of an organic compound with molecular formula C6H13 O. Assign chemical shift values to the H and c atoms of the compound. Find the structure. Show complete solutions. Predicted 1H NMR Spectrum ли 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 f1 (ppm)arrow_forward3. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-pentene. expanded structure: Condensed structure: Skeletal formula: 4. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-methyl-3-heptene. expanded structure: Condensed structure: Skeletal formula: following structurearrow_forwardPart IV. Propose a plausible Structure w/ the following descriptions: a) A 5-carbon hydrocarbon w/ a single peak in its proton decoupled the DEPT-135 Spectrum shows a negative peak C-NMR spectrum where b) what cyclohexane dione isomer gives the largest no. Of 13C NMR signals? c) C5H120 (5-carbon alcohol) w/ most deshielded carbon absent in any of its DEPT Spectivaarrow_forward
- 13C NMR is good for: a) determining the molecular weight of the compound b) identifying certain functional groups. c) determining the carbon skeleton, for example methyl vs ethyl vs propyl groups d) determining how many different kinds of carbon are in the moleculearrow_forward6 D 2. (1 pt) Limonene can be isolated by performing steam distillation of orange peel. Could you have performed this experiment using hexane instead of water? Explain. 3. (2 pts) Using GCMS results, analyze and discuss the purity of the Limonene obtained from the steam distillation of orange peel.arrow_forwardPart III. Arrange the following carbons (in blue) in order of increasing chemical shift. HO B NH 2 A CIarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

