
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter FRP, Problem 4P
Predict the products from each of the following reactions.
(a)
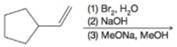
(b)
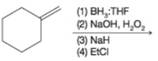
(c)
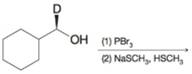
(d)
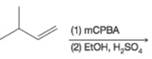
(e)
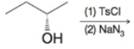
(f)

(g)
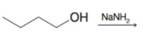
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Identify each chiral carbon as either R or S. Identify the overall carbohydrates as L or D
Ethers can be formed via acid-catalyzed acetal formation. Draw the mechanism for the molecule below and ethanol.
HOCH,
H
HO
CH-OH
OH H
OH
11
CH₂OH
F
II
OH H
H
0
+
H OH
Chapter FRP Solutions
Organic Chemistry
Ch. FRP - Prob. 1PCh. FRP - 2. Which member of these pairs is the more polar?...Ch. FRP - 5. Though they each contain only one type of...Ch. FRP - Predict the products from each of the following...Ch. FRP - Prob. 5PCh. FRP - Prob. 6PCh. FRP - Prob. 7PCh. FRP - Prob. 8PCh. FRP - Predict the products from each of the following...Ch. FRP - Prob. 10P
Ch. FRP - 13. Starting with propyne and using any other...Ch. FRP - Bromination of 2-methylbutane yields predominantly...Ch. FRP - An alkane (A) with the formula C6H14 reacts with...Ch. FRP - Prob. 14PCh. FRP - Prob. 15PCh. FRP - Dehydrohalogenation of meso-1, 2-dibromo-1,...Ch. FRP - Prob. 17PCh. FRP - Prob. 18PCh. FRP - 27. (R)-3-Methyl-1-pentene is treated separately...Ch. FRP - Prob. 20PCh. FRP - Prob. 21PCh. FRP - Prob. 22PCh. FRP - Prob. 23PCh. FRP - Synthesize the following compound by a method that...Ch. FRP - Provide three methods that employ Grignard...Ch. FRP - 34. Compound Yexhibits one NMR signal at (a...Ch. FRP - Prob. 27PCh. FRP - 36. Compound X shows a strong IR absorption band...Ch. FRP - Prob. 29PCh. FRP - 38. In addition to more highly fluorinated...Ch. FRP - Fluorination of (R)-2-flurobutane yields a mixture...Ch. FRP - Prob. 32PCh. FRP - Prob. 33P
Additional Science Textbook Solutions
Find more solutions based on key concepts
11. Birds and mammals are both endothermic, and both have four-chambered hearts. Most reptiles are ectothermic ...
Campbell Biology: Concepts & Connections (9th Edition)
Name the components (including muscles) of the thoracic cage. List the contents of the thorax.
Human Physiology: An Integrated Approach (8th Edition)
The number of named species is about __________, but the actual number of species on Earth is estimated to be a...
Biology: Life on Earth (11th Edition)
A de-superheater has a flow of ammonia of 1.5kg/s at 1000kPa,100C that is mixed with another flow of ammonia at...
Fundamentals Of Thermodynamics
What is the clinical significance of the xiphoid process?
Principles of Anatomy and Physiology
Some organizations are starting to envision a sustainable societyone in which each generation inherits sufficie...
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the mechanism for the formation of diol by starting with one pen and all in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forwardDraw the mechanism for the oxidation of 3-bromo-cyclohexan-1-ol.arrow_forwardConvert the following Fischer projection to Haworth projections. show work and show the arrows please.arrow_forward
- Draw the mechanism for the substitution reaction converting an alcohol into an alkyl halide. If chirality is important to the reaction include it.arrow_forwardWrite, in words three different reactions we can use to make an alcohol.arrow_forwardDraw the reduction mechanism for the reduction of the aldehyde.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY