
Concept explainers
(a)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
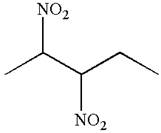
In this molecule, the longest continuous chain of carbon atoms has five carbon atoms. Hence, the root is pentane. The parent chain has two identical substituents; thus the chain is numbered so as to provide the lowest set of locants.
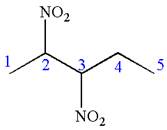
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(b)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms. The name of the substituent attached to the parent chain is written as a prefix to the left side of the root. The chain is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
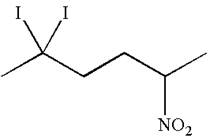
In this molecule, the longest continuous chain of carbon atoms has six carbon atoms. Hence, the root is hexane. The parent chain has three substituents; thus the chain is numbered so as to provide the lowest set of locants.
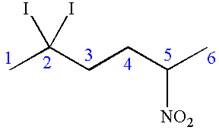
The substituents are arranged in an alphabetical order with respective locant.
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(c)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms. The name of the substituent attached to the parent chain is written as a prefix to the left side of the root. The chain is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
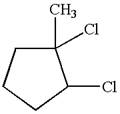
In this molecule, the five carbons ring is a parent. Hence, the root is cyclopentane. The ring has three substituents; thus, the ring is numbered so as to provide the lowest set of locants.
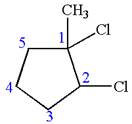
The substituents are arranged in alphabetical order with respective locant.
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(d)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms or the ring structure. The name of the substituent attached to the parent ring is written as a prefix to the left side of the root. The parent ring is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
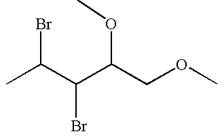
In this molecule, the five carbons ring is a parent. Hence, the root is cyclopentane. The parent chain has four substituents; thus, the chain is numbered so as to provide the lowest set of locants according to alphabetical order.
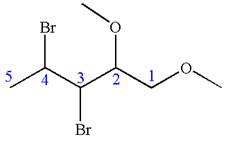
The substituents are arranged in alphabetical order with respective locant.
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(e)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms. The name of the substituent attached to the parent chain is written as a prefix to the left side of the root. The chain is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
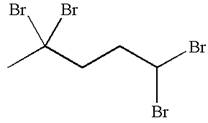
In this molecule, the five carbons ring is a parent. Hence, the root is cyclopentane. The parent chain has four identical substituents; thus, the chain is numbered so as to provide the lowest set of locants according to alphabetical order.
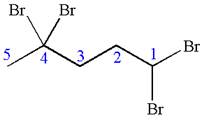
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(f)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms or the ring structure. The name of the substituent attached to the parent ring is written as a prefix to the left side of the root. The parent ring is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents in a way to provide the lowest set of locants.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
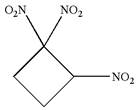
In this molecule, the four carbons ring is a parent. Hence, the root is cyclobutane. The ring has three identical substituents; thus, the ring is numbered so as to provide the lowest set of locants.
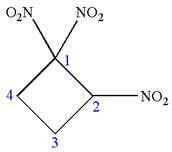
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(g)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms or the ring structure. The name of the substituent attached to the parent ring is written as a prefix to the left side of the root. The parent ring is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
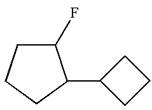
In this molecule, the five carbons ring is a parent. Hence, the root is cyclopentane. The ring has two substituents; thus, the ring is numbered so as to provide the lowest set of locants according to alphabetical order.
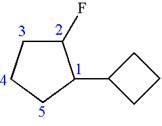
The substituents are arranged in alphabetical order with respective locant.
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(h)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms. The name of the substituent attached to the parent chain is written as a prefix to the left side of the root. The chain is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
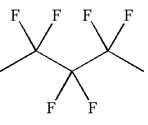
In this molecule, the longest continuous chain of carbon atoms has five carbon atoms. Hence, the root is pentane. The parent chain has six identical substituents; thus, the chain is numbered so as to provide the lowest set of locants.
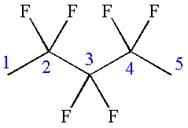
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(i)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms. The name of the substituent attached to the parent chain is written as a prefix to the left side of the root. The chain is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
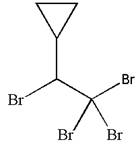
In this molecule, the longest continuous chain of carbon atoms has two carbon atoms. Hence, the root is ethane. The parent chain has five substituents; thus, the chain is numbered so as to provide the lowest set of locants.
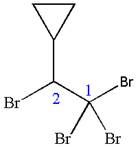
The substituents are arranged in alphabetical order with respective locant.
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(j)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms. The name of the substituent attached to the parent chain is written as a prefix to the left side of the root. The chain is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
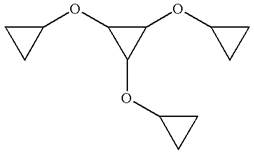
In this molecule, the middle ring of three carbons is a parent. Hence, the root is cyclopropane. The ring has three identical substituents.
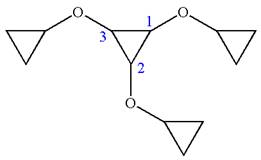
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
Want to see more full solutions like this?
Chapter A Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- 20. The Brusselator. This hypothetical system was first proposed by a group work- ing in Brussels [see Prigogine and Lefever (1968)] in connection with spatially nonuniform chemical patterns. Because certain steps involve trimolecular reac tions, it is not a model of any real chemical system but rather a prototype that has been studied extensively. The reaction steps are A-X. B+X-Y+D. 2X+ Y-3X, X-E. 305 It is assumed that concentrations of A, B, D, and E are kept artificially con stant so that only X and Y vary with time. (a) Show that if all rate constants are chosen appropriately, the equations de scribing a Brusselator are: dt A-(B+ 1)x + x²y, dy =Bx-x²y. diarrow_forwardProblem 3. Provide a mechanism for the following transformation: H₂SO A Me. Me Me Me Mearrow_forwardYou are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products: xi 1. ☑ 2. H₂O хе i Draw the missing reagent X you think will make this synthesis work in the drawing area below. If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. There is no reagent that will make this synthesis work without complications. : ☐ S ☐arrow_forward
- Predict the major products of this organic reaction: H OH 1. LiAlH4 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. G C टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new C-C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 CI MgCl ? Will the first product that forms in this reaction create a new CC bond? Yes No MgBr ? Will the first product that forms in this reaction create a new CC bond? Yes No G टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. དྲ。 ✗MgBr ? O CI Will the first product that forms in this reaction create a new C-C bond? Yes No • ? Will the first product that forms in this reaction create a new CC bond? Yes No × : ☐ Xarrow_forward
- Predict the major products of this organic reaction: OH NaBH4 H ? CH3OH Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. ☐ : Sarrow_forwardPredict the major products of this organic reaction: 1. LIAIHA 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. X : ☐arrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new C - C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 tu ? ? OH Will the first product that forms in this reaction create a new CC bond? Yes No Will the first product that forms in this reaction create a new CC bond? Yes No C $ ©arrow_forward
- As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C-C bond as its major product: 1. MgCl ? 2. H₂O* If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new CC bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new CC bond. G marrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M NH4 Ksp Hg2Br2 = 5.6×10-23.arrow_forwardgive example for the following(by equation) a. Converting a water insoluble compound to a soluble one. b. Diazotization reaction form diazonium salt c. coupling reaction of a diazonium salt d. indacator properties of MO e. Diazotization ( diazonium salt of bromobenzene)arrow_forward

 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax



