
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
3rd Edition
ISBN: 9780133858501
Author: Bruice
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 9.6, Problem 16P
- a. What is each ether’s systematic name?
- 1. CH3OCH2CH3
- 2. CH3CH2OCH2CH3
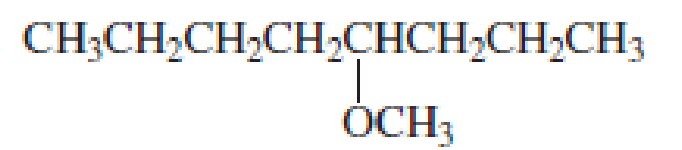
- 3. CH3CH2CH2OCH2CH2CH2CH3
- b. Do all of these ethers have common names?
- c. What are their common names?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
When 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.
When 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.
Which experimental number must be initialled by the Lab TA for the first run of Part 1 of the experiment?
a) the heat capacity of the calorimeter
b) Mass of sample
c) Ti
d) The molarity of the HCl
e) Tf
Predict products for the Following organic rxn/s by
writing the structurels of the correct products. Write
above the line provided"
your answer
D2
①CH3(CH2) 5 CH3 + D₂ (adequate)"
+
2
mited)
19
Spark
Spark
por every item.
4 CH 3 11
3 CH 3 (CH2) 4 C-H + CH3OH
CH2 CH3 + CH3 CH2OH
0
CH3
fou
+
KMnDy→
C43
+ 2 KMn Dy→→
C-OH
")
0
C-OH
1110
(4.)
9+3
=C
CH3
+ HNO 3
0
+ Heat>
+ CH3 C-OH + Heat
CH2CH3
- 3
2
+ D Heat H
3
CH 3 CH₂ CH₂ C = CH + 2 H₂ →
2
2
Chapter 9 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
Ch. 9.1 - Draw the structures of straight-chain alcohols...Ch. 9.1 - Prob. 2PCh. 9.1 - Prob. 3PCh. 9.2 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 9.2 - Prob. 5PCh. 9.2 - The observed relative reactivities of primary,...Ch. 9.4 - Which of the following alcohols would dehydrate...Ch. 9.4 - Prob. 10PCh. 9.4 - Prob. 11PCh. 9.4 - Prob. 12P
Ch. 9.4 - Prob. 13PCh. 9.5 - What product will be obtained from the reaction of...Ch. 9.5 - Prob. 15PCh. 9.6 - a. What is each ethers systematic name? 1....Ch. 9.8 - Draw the structure of the following: a....Ch. 9.8 - Prob. 20PCh. 9.8 - Would you expect the reactivity of a five-membered...Ch. 9.9 - Explain why the two arene oxides in Problem 22...Ch. 9.9 - Which compound is more likely to be...Ch. 9.11 - The following three nitrogen mustards were studied...Ch. 9 - What are the common and systematic names of the...Ch. 9 - Prob. 28PCh. 9 - Prob. 29PCh. 9 - Prob. 30PCh. 9 - Prob. 31PCh. 9 - What is the major product obtained from the...Ch. 9 - Draw structures for the following: a....Ch. 9 - Prob. 34PCh. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Prob. 37PCh. 9 - Ethylene oxide reacts readily with HO.because of...Ch. 9 - Propose a mechanism for each of the following...Ch. 9 - Which of the following ethers would be obtained in...Ch. 9 - Show how each of the following syntheses could be...Ch. 9 - Prob. 42PCh. 9 - Prob. 43PCh. 9 - Prob. 44PCh. 9 - Propose a mechanism for each of the following...Ch. 9 - a. Propose a mechanism for the following reaction:...Ch. 9 - Three arene oxides can be obtained from...Ch. 9 - Prob. 48PCh. 9 - The following reaction takes place several times...Ch. 9 - Show how each of the following compounds could be...Ch. 9 - Propose a mechanism for the following reaction:Ch. 9 - Propose a mechanism for the following reaction:Ch. 9 - What alkenes would you expect to be obtained from...Ch. 9 - Triethylenemelamine (TEM) is an antitumor agent....Ch. 9 - When a diol that has OH groups on adjacent carbons...Ch. 9 - What product is obtained when...Ch. 9 - Prob. 58P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forwardQ6: Using acetic acid as the acid, write the balanced chemical equation for the protonation of the two bases shown (on the -NH2). Include curved arrows to show the mechanism. O₂N- O₂N. -NH2 -NH2 a) Which of the two Bronsted bases above is the stronger base? Why? b) Identify the conjugate acids and conjugate bases for the reactants. c) Identify the Lewis acids and bases in the reactions.arrow_forwardQ5: For the two reactions below: a) Use curved electron-pushing arrows to show the mechanism for the reaction in the forward direction. Redraw the compounds to explicitly illustrate all bonds that are broken and all bonds that are formed. b) Label Bronsted acids and bases in the left side of the reactions. c) For reaction A, which anionic species is the weakest base? Which neutral compound is the stronger acid? Is the forward or reverse reaction favored? d) Label Lewis acids and bases, nucleophiles and electrophiles in the left side of the reactions. A. 용 CH3OH я хон CH3O OH B. HBr CH3ONa NaBr CH3OHarrow_forward
- potential energy Br b) Translate the Newman projection below to its wedge-and-dash drawing. F H. OH CH3 CI c) Isopentane (2-methylbutane) is a compound containing a branched carbon chain. Draw a Newman projection of six conformations about the C2-C3 bond of isopentane. On the curve of potential energy versus angle of internal rotation for isopentane, label each energy maximum and minimum with one of the conformations. 0° 。 F A B D C angle of internal rotation E F 360° (=0°) JDownlarrow_forwardQ7: Identify the functional groups in these molecules a) CH 3 b) Aspirin: HO 'N' Capsaicin HO O CH3 CH 3arrow_forwardQ2: Name the following alkanesarrow_forward
- 1. Complete the following table in your laboratory notebook. Substance Formula Methanol CH3OH Ethanol C2H5OH 1-Propanol C3H7OH 1-Butanol C4H9OH Pentane C5H12 Hexane C6H14 Water H₂O Acetone C3H60 Structural Formula Molecular Weight (g/mol) Hydrogen Bond (Yes or No)arrow_forwardQ1: Compare the relative acidity in each pair of compounds. Briefly explain. (a) CH3OH vs NH 3 (b) HF vs CH3COOH (c) NH3 vs CH4 (d) HCI vs HI (e) CH3COOH vs CH3SH (f) H₂C=CH2 vs CH3 CH3 (g) compare the acidity of the two bolded hydrogens O. H N- (h) compare the acidity of the two bolded hydrogens, draw resonance structures to explain H H Harrow_forwardQ3: Rank the following molecules in order of decreasing boiling point: (a) 3-methylheptane; (b) octane; (c) 2,4-dimethylhexane; (d) 2,2,4-trimethylpentane.arrow_forward
- Q5: Conformations of Alkanes a) Draw a Newman Projection of the compound below about the C2-C3 bond. H3C Cli... H IIIH Br CH3arrow_forwardThe ability of atoms to associate with each other depends ona) the electronic structure and its spatial orientation.b) the electron affinity.c) The other two answers are correct.arrow_forwardWhat is the final volume after you reach the final temperature? I put 1.73 but the answer is wrong not sure why The initial volume of gas is 1.60 LL , the initial temperature of the gas is 23.0 °C°C , and the system is in equilibrium with an external pressure of 1.2 bar (given by the sum of a 1 bar atmospheric pressure and a 0.2 bar pressure due to a brick that rests on top of the piston). Then, as you did in Exercise 1, you heat the gas slowly until the temperature reaches 48.2 °Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY