
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
3rd Edition
ISBN: 9780133858501
Author: Bruice
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 47P
Three arene oxides can be obtained from phenanthrene.
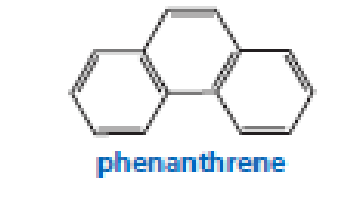
- a. Draw the structures of the three phenanthrene oxides.
- b. What phenols can be obtained from each phenanthrene oxide?
- c. If a phenanthrene oxide can lead to the formation of more than one phenol,which phenol is obtained in greater yield?
- d. Which of the three phenanthrene oxides is most likely to be carcinogenic?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A 45.0 mL solution containing a mixture of 0.0634 M KCN and 0.0634 M KCI is titrated with 0.107 M AgNO.
From this mixture, which silver salt will precipitate first? A list of Ksp values can be found in the table of solubility constants.
• AgCI
• not enough information to determine
AgCN
What is the concentration of Ag* at the first equivalence point?
[Ag*] =
Will the second silver salt begin to precipitate at the first equivalence point before the first silver salt has completely precipitated?
• not enough information to determine
• yes
• no
[Review Topics]
[References]
Indicate whether the pair of structures shown represent stereoisomers, constitutional isomers, different conformations of the
same compound, or the same conformation of a compound viewed from a different perspective.
Note that cis, trans isomers are an example of stereoisomers.
H₂N
✓ CI
H₂N
NH2
NH₂
CI
Submit Answer
Retry Entire Group
2 more group attempts remaining
Previous
Next>
Don't used Ai solution
Chapter 9 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
Ch. 9.1 - Draw the structures of straight-chain alcohols...Ch. 9.1 - Prob. 2PCh. 9.1 - Prob. 3PCh. 9.2 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 9.2 - Prob. 5PCh. 9.2 - The observed relative reactivities of primary,...Ch. 9.4 - Which of the following alcohols would dehydrate...Ch. 9.4 - Prob. 10PCh. 9.4 - Prob. 11PCh. 9.4 - Prob. 12P
Ch. 9.4 - Prob. 13PCh. 9.5 - What product will be obtained from the reaction of...Ch. 9.5 - Prob. 15PCh. 9.6 - a. What is each ethers systematic name? 1....Ch. 9.8 - Draw the structure of the following: a....Ch. 9.8 - Prob. 20PCh. 9.8 - Would you expect the reactivity of a five-membered...Ch. 9.9 - Explain why the two arene oxides in Problem 22...Ch. 9.9 - Which compound is more likely to be...Ch. 9.11 - The following three nitrogen mustards were studied...Ch. 9 - What are the common and systematic names of the...Ch. 9 - Prob. 28PCh. 9 - Prob. 29PCh. 9 - Prob. 30PCh. 9 - Prob. 31PCh. 9 - What is the major product obtained from the...Ch. 9 - Draw structures for the following: a....Ch. 9 - Prob. 34PCh. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Prob. 37PCh. 9 - Ethylene oxide reacts readily with HO.because of...Ch. 9 - Propose a mechanism for each of the following...Ch. 9 - Which of the following ethers would be obtained in...Ch. 9 - Show how each of the following syntheses could be...Ch. 9 - Prob. 42PCh. 9 - Prob. 43PCh. 9 - Prob. 44PCh. 9 - Propose a mechanism for each of the following...Ch. 9 - a. Propose a mechanism for the following reaction:...Ch. 9 - Three arene oxides can be obtained from...Ch. 9 - Prob. 48PCh. 9 - The following reaction takes place several times...Ch. 9 - Show how each of the following compounds could be...Ch. 9 - Propose a mechanism for the following reaction:Ch. 9 - Propose a mechanism for the following reaction:Ch. 9 - What alkenes would you expect to be obtained from...Ch. 9 - Triethylenemelamine (TEM) is an antitumor agent....Ch. 9 - When a diol that has OH groups on adjacent carbons...Ch. 9 - What product is obtained when...Ch. 9 - Prob. 58P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw resonance structures for the following compounds. Please provide a thorough explanation that allows for undertanding of topic.arrow_forwardBF3 has a no dipole moment. a) Draw the Lewis structure for BF3, showing all nonbonding electrons. b) Indicate the polarity of every atom in the structure using δ+ and δ– notation, and explain why the molecule has no net dipole. Please provide a thorough explanation that allows for undertanding of topic.arrow_forwardFor each reaction shown below follow the curved arrows to complete each equation by showing the structure of the products. Identify the acid, the base, the conjugated acid and conjugated base. Consutl a pKa table and choose the direciton the equilibrium goes. Please provide a thorough explanation that allows for undertanding of topic.arrow_forward
- Need help understanding please help Let’s assume the initial volume of the gas is 4.80 LL , the initial temperature of the gas is 29.0 °C°C , and the system is in equilibrium with an external pressure of 1.2 bar (given by the sum of a 1 bar atmospheric pressure and a 0.2 bar pressure due to a brick that rests on top of the piston). What is the final pressure of the gas? What is the final volume of the gas? What happens with the piston after you finish heating the gas? Assume you do not need to worry about the gas cooling down again because the outside of the container is at a lower temperature. That is, you manage to keep the gas at a constant temperature that equals 54.2 °C°C What is the sign of w? What is the value of w? Be careful with units. How do you convert bar*L to J?arrow_forwardFor a neutral hydrogen atom with an electron in the n = 4 state, how many different energies are possible when a photon is emitted?arrow_forwardFor the following compound identify the lone pairs and indicate if each lone pair is localized or delocalized. Please provide a thorough explanation that allows for undertanding of topic.arrow_forward
- What is the relationship between the following compounds? Choose between: (a)constitutional isomers, (b)resonance structures, (c)identical, (d) conformers Please provide a thorough explanation that allows for undertanding of topic.arrow_forwardCaffeine has the following structure. What is the hybridization state and molecular geometry at each nitrogen atom in Caffeine? Please provide a thorough explanation that allows for undertanding of topic.arrow_forwardWhat are the major products of the following reaction? Draw all the major products. If there are no major products, then there is no reaction that will take place. Use wedge and dash bonds when necessary.arrow_forward
- Tryptophan is an essential amino acid important in the synthesis of neurotransmitter serotonin in the body. What are the hybridization states, molecular geometry and approximate bond angle at the indicated carbon and nitrogen atoms? Please provide a thorough explanation that allows for undertanding of topic.arrow_forwardCan the target compound be efficiently synthesized in good yield from the substituted benzene of the starting material? If yes, draw the synthesis. Include all steps and all reactants.arrow_forwardWhat are the major products of this organic reaction? Please include all steps and explanations so that I can understand why. If there will be no significant reaction, explain why.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY