
Thermodynamics: An Engineering Approach
9th Edition
ISBN: 9781260048766
Author: CENGEL
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 9.12, Problem 97P
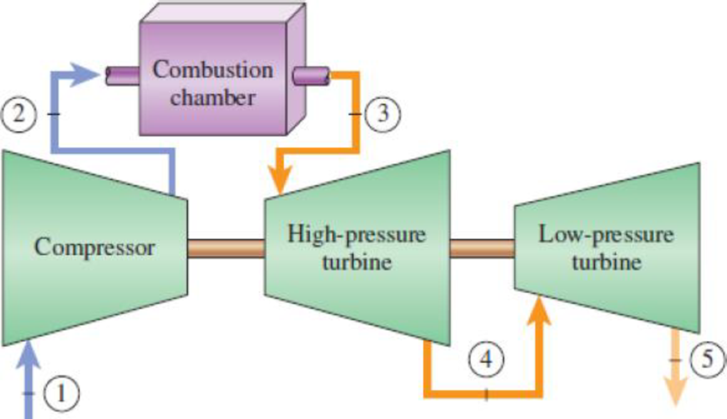
A gas-turbine power plant operates on a modified Brayton cycle shown in the figure with an overall pressure ratio of 8. Air enters the compressor at 0°C and 100 kPa. The maximum cycle temperature is 1500 K. The compressor and the turbines are isentropic. The high-pressure turbine develops just enough power to run the compressor. Assume constant properties for air at 300 K with cv = 0.718 kJ/kg·K, cp = 1.005 kJ/kg·K, R = 0.287 kJ/kg·K, k = 1.4.
- (a) Sketch the T-s diagram for the cycle. Label the data states.
- (b) Determine the temperature and pressure at state 4, the exit of the high-pressure turbine.
- (c) If the net power output is 200 MW, determine the mass flow rate of the air into the compressor in kg/s.
FIGURE P9–97
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Required information
A gas-turbine power plant operates on a modified Brayton cycle shown in the figure with an overall pressure ratio of 8. Air
enters the compressor at 0°C and 108 kPa. The maximum cycle temperature is 1500 K. The compressor and the turbines
are isentropic. The high-pressure turbine develops just enough power to run the compressor. Assume constant properties
for air at 300 K with cy=0.718 kJ/kg-K, cp=1005 kJ/kg-K. R=0.287 kJ/kg-K, and k=1.4.
Combustion
chamber
Compressor
to
High-pressure
Determine the temperature and pressure at state 4, the exit of the high-pressure turbine.
The temperature at state 4 is
The pressure at state 4 is [
K.
Low-pressure
kPa.
A gas-turbine power plant operates on a modified Brayton cycle with an
overall pressure ratio of 8. Air enters the compressor at 0°C and 100 kPa. The maximum
cycle temperature is 1500 K. The compressor and the turbines are isentropic. The high-
pressure turbine develops just enough power to run the compressor. Assume constant
properties for air at 300 K with C₁ = 0.718 kJ/kg K, Cp= 1.005 kJ/kg K, R = 0.287
kJ/kg K, k=1.4.
(a)
Sketch the (T-s) diagram for the cycle.
(b) Determine the temperature and pressure at state 4, the exit of the high-pressure turbine.
(c) If the net power output is 200 MW, determine the mass flow rate of the air into the
compressor, in kg/s.
Exhaust gases from the turbine of a simple Brayton cycle are quite hot and may be used for other thermal purposes. One proposed use is generating saturated steam at 110C from water at 30C in a boiler. This steam will be distributed to several buildings on a college campus for space heating. A Brayton cycle with a pressure ratio of 6 is to be used for this purpose. Plot the power produced, the flow rate of produced steam, and the maximum cycle temperature as functions of the rate at which heat is added to the cycle. The temperature at the turbine inlet is not to exceed 2000C.
Chapter 9 Solutions
Thermodynamics: An Engineering Approach
Ch. 9.12 - What are the air-standard assumptions?Ch. 9.12 - What is the difference between air-standard...Ch. 9.12 - Prob. 3PCh. 9.12 - How does the thermal efficiency of an ideal cycle,...Ch. 9.12 - How are the combustion and exhaust processes...Ch. 9.12 - What does the area enclosed by the cycle represent...Ch. 9.12 - Prob. 7PCh. 9.12 - Can the mean effective pressure of an automobile...Ch. 9.12 - What is the difference between spark-ignition and...Ch. 9.12 - Prob. 10P
Ch. 9.12 - Prob. 11PCh. 9.12 - Can any ideal gas power cycle have a thermal...Ch. 9.12 - Prob. 13PCh. 9.12 - Prob. 14PCh. 9.12 - Prob. 15PCh. 9.12 - Prob. 16PCh. 9.12 - Prob. 17PCh. 9.12 - Prob. 18PCh. 9.12 - Prob. 19PCh. 9.12 - Repeat Prob. 919 using helium as the working...Ch. 9.12 - The thermal energy reservoirs of an ideal gas...Ch. 9.12 - Consider a Carnot cycle executed in a closed...Ch. 9.12 - Consider a Carnot cycle executed in a closed...Ch. 9.12 - What four processes make up the ideal Otto cycle?Ch. 9.12 - Are the processes that make up the Otto cycle...Ch. 9.12 - How do the efficiencies of the ideal Otto cycle...Ch. 9.12 - How does the thermal efficiency of an ideal Otto...Ch. 9.12 - Why are high compression ratios not used in...Ch. 9.12 - An ideal Otto cycle with a specified compression...Ch. 9.12 - Prob. 30PCh. 9.12 - Prob. 31PCh. 9.12 - Determine the mean effective pressure of an ideal...Ch. 9.12 - Reconsider Prob. 932E. Determine the rate of heat...Ch. 9.12 - An ideal Otto cycle has a compression ratio of 8....Ch. 9.12 - Prob. 36PCh. 9.12 - A spark-ignition engine has a compression ratio of...Ch. 9.12 - An ideal Otto cycle has a compression ratio of 7....Ch. 9.12 - Prob. 39PCh. 9.12 - An ideal Otto cycle with air as the working fluid...Ch. 9.12 - Repeat Prob. 940E using argon as the working...Ch. 9.12 - Someone has suggested that the air-standard Otto...Ch. 9.12 - Repeat Prob. 942 when isentropic processes are...Ch. 9.12 - Prob. 44PCh. 9.12 - Prob. 45PCh. 9.12 - Prob. 46PCh. 9.12 - Prob. 47PCh. 9.12 - Prob. 48PCh. 9.12 - Prob. 49PCh. 9.12 - Prob. 50PCh. 9.12 - Prob. 51PCh. 9.12 - Prob. 52PCh. 9.12 - Prob. 53PCh. 9.12 - Prob. 54PCh. 9.12 - Prob. 55PCh. 9.12 - Prob. 56PCh. 9.12 - Prob. 57PCh. 9.12 - Repeat Prob. 957, but replace the isentropic...Ch. 9.12 - Prob. 60PCh. 9.12 - Prob. 61PCh. 9.12 - The compression ratio of an ideal dual cycle is...Ch. 9.12 - Repeat Prob. 962 using constant specific heats at...Ch. 9.12 - Prob. 65PCh. 9.12 - Prob. 66PCh. 9.12 - Prob. 67PCh. 9.12 - An air-standard cycle, called the dual cycle, with...Ch. 9.12 - Prob. 69PCh. 9.12 - Prob. 70PCh. 9.12 - Consider the ideal Otto, Stirling, and Carnot...Ch. 9.12 - Consider the ideal Diesel, Ericsson, and Carnot...Ch. 9.12 - An ideal Ericsson engine using helium as the...Ch. 9.12 - An ideal Stirling engine using helium as the...Ch. 9.12 - Prob. 75PCh. 9.12 - Prob. 76PCh. 9.12 - Prob. 77PCh. 9.12 - Prob. 78PCh. 9.12 - Prob. 79PCh. 9.12 - For fixed maximum and minimum temperatures, what...Ch. 9.12 - What is the back work ratio? What are typical back...Ch. 9.12 - Why are the back work ratios relatively high in...Ch. 9.12 - How do the inefficiencies of the turbine and the...Ch. 9.12 - A simple ideal Brayton cycle with air as the...Ch. 9.12 - A stationary gas-turbine power plant operates on a...Ch. 9.12 - A gas-turbine power plant operates on the simple...Ch. 9.12 - Prob. 87PCh. 9.12 - Prob. 88PCh. 9.12 - Repeat Prob. 988 when the isentropic efficiency of...Ch. 9.12 - Repeat Prob. 988 when the isentropic efficiency of...Ch. 9.12 - Repeat Prob. 988 when the isentropic efficiencies...Ch. 9.12 - Air is used as the working fluid in a simple ideal...Ch. 9.12 - An aircraft engine operates on a simple ideal...Ch. 9.12 - Repeat Prob. 993 for a pressure ratio of 15.Ch. 9.12 - A gas-turbine power plant operates on the simple...Ch. 9.12 - A simple ideal Brayton cycle uses argon as the...Ch. 9.12 - A gas-turbine power plant operates on a modified...Ch. 9.12 - A gas-turbine power plant operating on the simple...Ch. 9.12 - Prob. 99PCh. 9.12 - Prob. 100PCh. 9.12 - Prob. 101PCh. 9.12 - Prob. 102PCh. 9.12 - Prob. 103PCh. 9.12 - Prob. 104PCh. 9.12 - A gas turbine for an automobile is designed with a...Ch. 9.12 - Rework Prob. 9105 when the compressor isentropic...Ch. 9.12 - A gas-turbine engine operates on the ideal Brayton...Ch. 9.12 - An ideal regenerator (T3 = T5) is added to a...Ch. 9.12 - Prob. 109PCh. 9.12 - Prob. 111PCh. 9.12 - A Brayton cycle with regeneration using air as the...Ch. 9.12 - Prob. 113PCh. 9.12 - Prob. 114PCh. 9.12 - Prob. 115PCh. 9.12 - Prob. 116PCh. 9.12 - Prob. 117PCh. 9.12 - Prob. 118PCh. 9.12 - Prob. 119PCh. 9.12 - Prob. 120PCh. 9.12 - A simple ideal Brayton cycle without regeneration...Ch. 9.12 - A simple ideal Brayton cycle is modified to...Ch. 9.12 - Consider a regenerative gas-turbine power plant...Ch. 9.12 - Repeat Prob. 9123 using argon as the working...Ch. 9.12 - Consider an ideal gas-turbine cycle with two...Ch. 9.12 - Repeat Prob. 9125, assuming an efficiency of 86...Ch. 9.12 - A gas turbine operates with a regenerator and two...Ch. 9.12 - Prob. 128PCh. 9.12 - Prob. 129PCh. 9.12 - Prob. 130PCh. 9.12 - Prob. 131PCh. 9.12 - Air at 7C enters a turbojet engine at a rate of 16...Ch. 9.12 - Prob. 133PCh. 9.12 - A turbojet is flying with a velocity of 900 ft/s...Ch. 9.12 - A pure jet engine propels an aircraft at 240 m/s...Ch. 9.12 - A turbojet aircraft is flying with a velocity of...Ch. 9.12 - Prob. 137PCh. 9.12 - Prob. 138PCh. 9.12 - Reconsider Prob. 9138E. How much change would...Ch. 9.12 - Consider an aircraft powered by a turbojet engine...Ch. 9.12 - An ideal Otto cycle has a compression ratio of 8....Ch. 9.12 - An air-standard Diesel cycle has a compression...Ch. 9.12 - Prob. 144PCh. 9.12 - Prob. 145PCh. 9.12 - Prob. 146PCh. 9.12 - Prob. 147PCh. 9.12 - A Brayton cycle with regeneration using air as the...Ch. 9.12 - Prob. 150PCh. 9.12 - A gas turbine operates with a regenerator and two...Ch. 9.12 - A gas-turbine power plant operates on the...Ch. 9.12 - Prob. 153PCh. 9.12 - An air-standard cycle with variable specific heats...Ch. 9.12 - Prob. 155RPCh. 9.12 - Prob. 156RPCh. 9.12 - Prob. 157RPCh. 9.12 - Prob. 158RPCh. 9.12 - Prob. 159RPCh. 9.12 - Prob. 160RPCh. 9.12 - Prob. 161RPCh. 9.12 - Consider an engine operating on the ideal Diesel...Ch. 9.12 - Repeat Prob. 9162 using argon as the working...Ch. 9.12 - Prob. 164RPCh. 9.12 - Prob. 165RPCh. 9.12 - Prob. 166RPCh. 9.12 - Prob. 167RPCh. 9.12 - Consider an ideal Stirling cycle using air as the...Ch. 9.12 - Prob. 169RPCh. 9.12 - Consider a simple ideal Brayton cycle with air as...Ch. 9.12 - Prob. 171RPCh. 9.12 - A Brayton cycle with a pressure ratio of 15...Ch. 9.12 - Helium is used as the working fluid in a Brayton...Ch. 9.12 - Consider an ideal gas-turbine cycle with one stage...Ch. 9.12 - Prob. 176RPCh. 9.12 - Prob. 177RPCh. 9.12 - Prob. 180RPCh. 9.12 - Prob. 181RPCh. 9.12 - Prob. 182RPCh. 9.12 - For specified limits for the maximum and minimum...Ch. 9.12 - A Carnot cycle operates between the temperature...Ch. 9.12 - Prob. 194FEPCh. 9.12 - Prob. 195FEPCh. 9.12 - Helium gas in an ideal Otto cycle is compressed...Ch. 9.12 - Prob. 197FEPCh. 9.12 - Prob. 198FEPCh. 9.12 - In an ideal Brayton cycle, air is compressed from...Ch. 9.12 - In an ideal Brayton cycle, air is compressed from...Ch. 9.12 - Consider an ideal Brayton cycle executed between...Ch. 9.12 - An ideal Brayton cycle has a net work output of...Ch. 9.12 - In an ideal Brayton cycle with regeneration, argon...Ch. 9.12 - In an ideal Brayton cycle with regeneration, air...Ch. 9.12 - Consider a gas turbine that has a pressure ratio...Ch. 9.12 - An ideal gas turbine cycle with many stages of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A simple ideal Brayton cycle operates with air with minimum and maximum temperatures of 27°C and 877°C. It is designed so that the maximum cycle pressure is 2000 kPa and the minimum cycle pressure is 100 kPa. Determine the net work produced per unit mass of air each time this cycle is executed and the cycle's thermal efficiency. Use constant specific heats at room temperature. The properties of air at room temperature are cp=1.005 kJ/kg-K and k = 1.4. 2 Compressor 0 Qout lin Turbine W The net work produced per unit mass of air is The thermal efficiency is %. kJ/kg. harrow_forwardAn ideal Brayton cycle has a pressure ratio of 7:1. The temperature of the air is 30 UC at the inlet to the compressor and 1,001 °C at the inlet to the turbine. Take Specific heat at constant pressure and constant volume for air as 1.006 kJ/kg K and 0.717 kJ/kg K respectively. Calculate the work ratio of the plant to three decimal places.arrow_forwardA simple ideal Brayton cycle operates with air with minimum and maximum temperatures of 27°C and 727°C. It is designed so that the maximum cycle pressure is 2000 kPa and the minimum cycle pressure is 100 kPa. Determine the net work produced per unit mass of air each time this cycle is executed and the cycle’s thermal efficiency. Use constant specific heats at room temperaturearrow_forward
- Consider a simple ideal Brayton cycle with air as the working fluid. The pressure ratio of the cycle is 7.2, and the minimum and maximum temperatures are 300 K and 1350 K, respectively. Now the pressure ratio is doubled without changing the minimum and the maximum temperatures in the cycle. Assuming constant specific heats for air at room temperature; show the initial process and process change in a T-s diagram; calculate the change in the net work output per unit mass; and determine the change in thermal efficiency of the cycle.arrow_forward5. A large stationary Brayton cycle gas-turbine power plant delivers a power output of 100 MW to an electric generator. The minimum temperature in the cycle is 300 K, and the maximum temperature is 1600 K. The minimum pressure in the cycle is 100 kPa, and the compressor pressure ratio is 14 to 1. Calculate the power output of the turbine. What fraction of the turbine output is required to drive the compressor? What is the thermal efficiency of the cycle?arrow_forwardI need the answer as soon as possiblearrow_forward
- For an air-standard Brayton cycle the air flow rate entering the compressor is 10 m3/s at 300 K and 100 kPa. The pressure ratio is 12, and the cycle maximum temperature is 1100 K. Determine (a) the net power developed; (b) the cycle thermal efficiency.arrow_forwardConsider a simple Brayton cycle that uses air as the working fluid to operate a gas-turbine power plant between the pressure limits of 100kPa and 900kPa. Air enters the compressor at 298K and leaves at 610K at a mass flow rate of 1200kg/min. The maximum cycle temperature is 1380K. The net power output from the cycle is measured experimentally to be 55 MW. Assuming constant properties (Cv,Cp, R, and k) for air at 300K: a) Sketch a T-s diagram for the cycle b) Determine the isentropic efficiency of the turbine c) Determine the thermal efficiencyarrow_forwardIn an ideal gas turbine cycle with two-stage compression and expansion, the pressure ratio in both stages of the compressor and turbine is 3.8. The air enters both stages of the compressor at 300 K and both stages of the turbine at 1069 K. What will be the thermal efficiency of the cycle if a regenerator with 75% efficiency is used.arrow_forward
- Consider a Brayton cycle with a pressure ratio of 13, that receives 6.5 lbm/s of air at 75 ∘F. Assume that the maximum allowable temperature in the machinery is 2500 ∘F. If the isentropic efficiency of the compressor and turbine is 93 % and 92 % respectively, then what is the thermal efficiency of this system? heat capacity of air: c_p = 0.240 btu/(R*lbm) k of air is 1.4arrow_forwardRequired information Problem 09.097 - Modified Brayton Cycle with Two-Step Expansion - DEPENDENT MULTI-PART PROBLEM - ASSIGN ALL PARTS A gas-turbine power plant operates on a modified Brayton cycle shown in the figure with an overall pressure ratio of 8. Air enters the compressor at 0°C and 110 kPa. The maximum cycle temperature is 1500 K. The compressor and the turbines are isentropic. The high-pressure turbine develops just enough power to run the compressor. Assume constant properties for air at 300 K with cy= 0.718 kJ/kg-K, cp=1.005 kJ/kg-K, R= 0.287 kJ/kg-K, and k = 1.4. Combustion chamber Compressor to High-pressure turbine Low-pressure turbine Problem 09.097.c - Net Power Output of Low Pressure Turbine If the net power output is 200 MW, determine the mass flow rate of the air into the compressor in kg/s. The mass flow rate of the air into the compressor is kg/s.arrow_forwardRequired information Problem 09.097 - Modified Brayton Cycle with Two-Step Expansion - DEPENDENT MULTI-PART PROBLEM - ASSIGN ALL PARTS A gas-turbine power plant operates on a modified Brayton cycle shown in the figure with an overall pressure ratio of 8. Air enters the compressor at 0°C and 110 kPa. The maximum cycle temperature is 1500 K. The compressor and the turbines are isentropic. The high-pressure turbine develops just enough power to run the compressor. Assume constant properties for air at 300 K with cy= 0.718 kJ/kg-K, cp= 1.005 kJ/kg-K, R= 0.287 kJ/kg-K, and k = 1.4. Combustion chamber Compressor O High-pressure turbine Problem 09.097.b - Temperature and Pressure After High Pressure Turbine The temperature at state 4 is The pressure at state 4 is Determine the temperature and pressure at state 4, the exit of the high-pressure turbine. (You must provide an answer before moving on to the next part.) Low-pressure turbine K. kPaarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Power Plant Explained | Working Principles; Author: RealPars;https://www.youtube.com/watch?v=HGVDu1z5YQ8;License: Standard YouTube License, CC-BY